Systematic review with meta-analysis: association between vedolizumab trough concentration and clinical outcomes in patients with inflammatory bowel diseases
- PMID: 31483522
- PMCID: PMC7083298
- DOI: 10.1111/apt.15484
Systematic review with meta-analysis: association between vedolizumab trough concentration and clinical outcomes in patients with inflammatory bowel diseases
Abstract
Background: There has been limited evaluation of the association between vedolizumab trough concentration and clinical outcomes in patients with inflammatory bowel diseases (IBD).
Aim: To perform a systematic review and meta-analysis to evaluate the potential role of therapeutic drug monitoring (TDM) for vedolizumab.
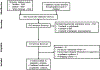
Methods: Through a systematic literature search through 28 February 2019, we identified five cohort studies (558 patients, 42% with ulcerative colitis) reporting the association between vedolizumab trough concentration and clinical outcomes in patients with IBD. We calculated mean difference (MD) in vedolizumab trough concentration in patients achieving vs not achieving clinical outcomes, and qualitatively synthesized thresholds associated with favourable outcomes.
Results: In patients with UC, median vedolizumab trough concentrations were consistently higher in patients achieving clinical remission (median, 14.3 μg/mL vs 10.5 μg/mL; MD, 5.1 μg/mL, 95% CI, 2.8-7.4) or endoscopic remission (median, 13.0 μg/mL vs 9.7; MD, 5.1 μg/mL, 95% CI, 2.2-7.9). In patients with CD, there was no significant difference in median vedolizumab trough concentrations in patients achieving vs not achieving clinical remission (MD, 2.0 μg/mL; 95% CI, -0.5 to 4.5) or endoscopic remission (MD, 3.6 μg/mL; 95% CI, -1.4 to 8.6). In patients with UC, week 6 vedolizumab trough concentrations ≥18.5-20.8 μg/mL, and maintenance trough concentrations ≥9.0-12.6 μg/mL were associated with favourable clinical outcomes. Antibodies to vedolizumab were reported in 1.7%-3.0% patients on maintenance therapy.
Conclusion: Based on meta-analysis, patients with UC who achieve endoscopic and clinical remission have significantly higher vedolizumab trough concentration during maintenance therapy. Vedolizumab trough concentration >20 μg/mL at week 6, and >12 μg/mL during maintenance may be associated with better outcomes, though cause-effect relationship remains unclear. Prospective studies on reactive and proactive therapeutic drug monitoring of vedolizumab (vs empiric dose escalation) are warranted.
© 2019 John Wiley & Sons Ltd.
Figures
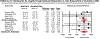
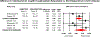

References
-
- Khanna R, Sattin BD, Afif W, et al. Review article: a clinician’s guide for therapeutic drug monitoring of infliximab in inflammatory bowel disease. Aliment Pharmacol Ther 2013;38(5):447–59. - PubMed
-
- Mitrev N, Vande Casteele N, Seow CH, et al. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2017;46(11–12):1037–1053. - PubMed
-
- Ordas I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012;10(10):1079–87; quiz e85–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
