Ultrafast magnetic resonance spectroscopic imaging using SPICE with learned subspaces
- PMID: 31483526
- PMCID: PMC6824949
- DOI: 10.1002/mrm.27980
Ultrafast magnetic resonance spectroscopic imaging using SPICE with learned subspaces
Abstract
Purpose: To develop a subspace learning method for the recently proposed subspace-based MRSI approach known as SPICE, and achieve ultrafast 1 H-MRSI of the brain.
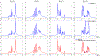
Theory and methods: A novel strategy is formulated to learn a low-dimensional subspace representation of MR spectra from specially acquired training data and use the learned subspace for general MRSI experiments. Specifically, the subspace learning problem is formulated as learning "empirical" distributions of molecule-specific spectral parameters (e.g., concentrations, lineshapes, and frequency shifts) by integrating physics-based model and the training data. The learned spectral parameters and quantum mechanical simulation basis can then be combined to construct acquisition-specific subspace for spatiospectral encoding and processing. High-resolution MRSI acquisitions combining ultrashort-TE/short-TR excitation, sparse sampling, and the elimination of water suppression have been performed to evaluate the feasibility of the proposed method.
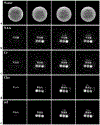
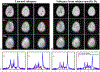
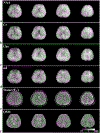
Results: The accuracy of the learned subspace and the capability of the proposed method in producing high-resolution 3D 1 H metabolite maps and high-quality spatially resolved spectra (with a nominal resolution of ∼2.4 × 2.4 × 3 mm3 in 5 minutes) were demonstrated using phantom and in vivo studies. By eliminating water suppression, we are also able to extract valuable information from the water signals for data processing ( map, frequency drift, and coil sensitivity) as well as for mapping tissue susceptibility and relaxation parameters.
Conclusions: The proposed method enables ultrafast 1 H-MRSI of the brain using a learned subspace, eliminating the need of acquiring subject-dependent navigator data (known as ) in the original SPICE technique. It represents a new way to perform MRSI experiments and an important step toward practical applications of high-resolution MRSI.
Keywords: MR spectroscopic imaging; no water suppression; rapid spatiospectral encoding; subspace learning; union-of-subspaces model.
© 2019 International Society for Magnetic Resonance in Medicine.
Figures
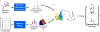

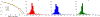
Similar articles
-
High-resolution, volumetric diffusion-weighted MR spectroscopic imaging of the brain.Magn Reson Med. 2025 Aug;94(2):450-469. doi: 10.1002/mrm.30479. Epub 2025 Mar 10. Magn Reson Med. 2025. PMID: 40065541 Free PMC article.
-
Simultaneous QSM and metabolic imaging of the brain using SPICE.Magn Reson Med. 2018 Jan;79(1):13-21. doi: 10.1002/mrm.26972. Epub 2017 Oct 24. Magn Reson Med. 2018. PMID: 29067730 Free PMC article.
-
High-resolution (1) H-MRSI of the brain using SPICE: Data acquisition and image reconstruction.Magn Reson Med. 2016 Oct;76(4):1059-70. doi: 10.1002/mrm.26019. Epub 2015 Oct 28. Magn Reson Med. 2016. PMID: 26509928 Free PMC article.
-
Multi-echo acquisition.Neuroimage. 2012 Aug 15;62(2):665-71. doi: 10.1016/j.neuroimage.2011.10.057. Epub 2011 Oct 25. Neuroimage. 2012. PMID: 22056458 Free PMC article. Review.
-
Accelerated MR spectroscopic imaging-a review of current and emerging techniques.NMR Biomed. 2021 May;34(5):e4314. doi: 10.1002/nbm.4314. Epub 2020 May 12. NMR Biomed. 2021. PMID: 32399974 Free PMC article. Review.
Cited by
-
High-resolution, volumetric diffusion-weighted MR spectroscopic imaging of the brain.Magn Reson Med. 2025 Aug;94(2):450-469. doi: 10.1002/mrm.30479. Epub 2025 Mar 10. Magn Reson Med. 2025. PMID: 40065541 Free PMC article.
-
Subnormothermic Ex Vivo Porcine Kidney Perfusion Improves Energy Metabolism: Analysis Using 31P Magnetic Resonance Spectroscopic Imaging.Transplant Direct. 2022 Sep 26;8(10):e1354. doi: 10.1097/TXD.0000000000001354. eCollection 2022 Oct. Transplant Direct. 2022. PMID: 36176724 Free PMC article.
-
Achieving high-resolution 1H-MRSI of the human brain with compressed-sensing and low-rank reconstruction at 7 Tesla.J Magn Reson. 2021 Oct;331:107048. doi: 10.1016/j.jmr.2021.107048. Epub 2021 Aug 11. J Magn Reson. 2021. PMID: 34438355 Free PMC article.
-
High-Dimensional MR Spatiospectral Imaging by Integrating Physics-Based Modeling and Data-Driven Machine Learning: Current progress and future directions.IEEE Signal Process Mag. 2023 Mar;40(2):101-115. doi: 10.1109/msp.2022.3203867. Epub 2023 Feb 27. IEEE Signal Process Mag. 2023. PMID: 37538148 Free PMC article.
-
Ultrafast J-resolved magnetic resonance spectroscopic imaging for high-resolution metabolic brain imaging.Nat Biomed Eng. 2025 Jun 20. doi: 10.1038/s41551-025-01418-4. Online ahead of print. Nat Biomed Eng. 2025. PMID: 40542105
References
-
- Lauterbur PC, Kramer DM, House WV, Chen CN, Zeugmatographic high resolution nuclear magnetic resonance spectroscopy: Images of chemical inhomogeneity within macroscopic objects. J Amer Chem Soc 1975;97:6866 – 6868.
-
- Maudsley AA, Hilal SK, Perman WH, Simon HE, Spatially resolved high resolution spectroscopy by “four-dimensional” NMR. J Magn Reson 1983;51:147 – 152.
-
- Luyten PR, Marien AJ, Heindel W, van Gerwen PH, Herholz K, den Hollander JA, Friedmann G, Heiss WD, Metabolic imaging of patients with intracranial tumors: H-1 MR spectroscopic imaging and PET. Radiology 1990;176:791 – 799. - PubMed
-
- de Graaf RA, In Vivo NMR Spectroscopy: Principles and Techniques. Hoboken, NJ: John Wiley and Sons, 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

