β-Hydroxy-Stabilized Boron-Nitrogen Heterocycles Enable Rapid and Efficient C-Terminal Protein Modification
- PMID: 31483610
- PMCID: PMC7354006
- DOI: 10.1021/acs.bioconjchem.9b00534
β-Hydroxy-Stabilized Boron-Nitrogen Heterocycles Enable Rapid and Efficient C-Terminal Protein Modification
Abstract
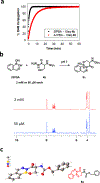
Bioorthogonal chemistry has enabled the development of bioconjugates in physiological environments while averting interference from endogenous biomolecules. Reactions between carbonyl-containing molecules and alkoxyamines or hydrazines have experienced a resurgence in popularity in bioorthogonal chemistry owing to advances that allow the reactions to occur under physiological conditions. In particular, ortho-carbonyl-substituted phenylboronic acids (CO-PBAs) exhibit greatly accelerated rates of hydrazone and oxime formation via intramolecular Lewis acid catalysis. Unfortunately, the rate of the reverse reaction is also increased, yielding a kinetically less stable bioconjugate. When the substrate is a hydrazine derivative, an intramolecular reaction between the boronic acid and the hydrazone can lead to the formation of a heterocycle containing a boron-nitrogen bond. We have shown previously that α-amino hydrazides undergo rapid reaction with CO-PBAs to form highly stable, tricyclic products, and that this reaction is orthogonal to the popular azide-alkyne and tetrazine-alkene reactions. In this work, we explore a series of heteroatom-substituted hydrazides for their ability to form tricyclic products with two CO-PBAs, 2-formylphenylboronic acid (2fPBA), and 2-acetylphenylboronic acid (AcPBA). In particular, highly stable products were formed using β-hydroxy hydrazides and 2fPBA. C-Terminal β-hydroxy hydrazide proteins are available using conventional biochemical methods, which alleviates one of the difficulties with applications of bioorthogonal chemical reactions: site-specific incorporation of a reactive group into the biomolecular target. Using sortase-mediated ligation (SML), C-terminal threonine and serine hydrazides were appended to a model eGFP protein in high yield. Subsequent labeling with 2fPBA functionalized probes could be performed quickly and quantitatively at neutral pH using micromolar concentrations of reactants. The SML process was applied directly to an expressed protein in cellular extract, and the C-terminal modified target protein was selectively immobilized using 2fPBA-agarose. Elution from the agarose yielded a highly pure protein that retained the hydrazide functionality. This strategy should be generally applicable for rapid, efficient site-specific protein labeling, protein immobilization, and preparation of highly pure functionalized proteins.
Conflict of interest statement
The authors declare the following competing financial interest(s): S.L.B is an inventor on a patent pertaining to this chemistry. The other authors declare no competing financial interest.
Figures




Similar articles
-
Boron enabled bioconjugation chemistries.Chem Soc Rev. 2024 Dec 9;53(24):11888-11907. doi: 10.1039/d4cs00750f. Chem Soc Rev. 2024. PMID: 39479937 Free PMC article. Review.
-
Site-Specific Bioconjugation and Multi-Bioorthogonal Labeling via Rapid Formation of a Boron-Nitrogen Heterocycle.Bioconjug Chem. 2019 May 15;30(5):1554-1564. doi: 10.1021/acs.bioconjchem.9b00246. Epub 2019 May 3. Bioconjug Chem. 2019. PMID: 31026151 Free PMC article.
-
Formation of hydrazones and stabilized boron-nitrogen heterocycles in aqueous solution from carbohydrazides and ortho-formylphenylboronic acids.Org Biomol Chem. 2017 Sep 20;15(36):7543-7548. doi: 10.1039/c7ob01708a. Org Biomol Chem. 2017. PMID: 28853481 Free PMC article.
-
Merging Boron with Nitrogen-Oxygen Bonds: A Review on BON Heterocycles.Top Curr Chem (Cham). 2021 Feb 5;379(2):8. doi: 10.1007/s41061-020-00317-3. Top Curr Chem (Cham). 2021. PMID: 33544252 Review.
-
Bioorthogonal chemistry for site-specific labeling and surface immobilization of proteins.Acc Chem Res. 2011 Sep 20;44(9):762-73. doi: 10.1021/ar200046h. Epub 2011 Jun 7. Acc Chem Res. 2011. PMID: 21648407
Cited by
-
Boron enabled bioconjugation chemistries.Chem Soc Rev. 2024 Dec 9;53(24):11888-11907. doi: 10.1039/d4cs00750f. Chem Soc Rev. 2024. PMID: 39479937 Free PMC article. Review.
-
Regioselective alkylation of 2,4-dihydroxybenzyaldehydes and 2,4-dihydroxyacetophenones.Tetrahedron Lett. 2022 Apr 13;95:153755. doi: 10.1016/j.tetlet.2022.153755. Epub 2022 Mar 23. Tetrahedron Lett. 2022. PMID: 35495552 Free PMC article.
-
Therapeutic Oligonucleotides: An Outlook on Chemical Strategies to Improve Endosomal Trafficking.Cells. 2023 Sep 11;12(18):2253. doi: 10.3390/cells12182253. Cells. 2023. PMID: 37759475 Free PMC article. Review.
-
Iminoboronates as Dual-Purpose Linkers in Chemical Probe Development.Chemistry. 2021 Feb 15;27(10):3292-3296. doi: 10.1002/chem.202005115. Epub 2021 Jan 14. Chemistry. 2021. PMID: 33259638 Free PMC article.
-
Protein-Nucleic Acid Conjugation with Sterol Linkers Using Hedgehog Autoprocessing.Bioconjug Chem. 2019 Nov 20;30(11):2799-2804. doi: 10.1021/acs.bioconjchem.9b00550. Epub 2019 Oct 10. Bioconjug Chem. 2019. PMID: 31600061 Free PMC article.
References
-
- Lang K; Chin JW Bioorthogonal Reactions for Labeling Proteins. ACS Chem. Biol 2014, 9, 16–20. - PubMed
-
- Debets MF; Van Berkel SS; Dommerholt J; Dirks AJ; Rutjes FPJT; Van Delft FL Bioconjugation with Strained Alkenes and Alkynes. Acc. Chem. Res 2011, 44, 805–815. - PubMed
-
- Knall A-C; Slugovc C Inverse Electron Demand Diels–Alder (IEDDA)-Initiated Conjugation: A (High) Potential Click Chemistry Scheme. Chem. Soc. Rev 2013, 42, 5131–5142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

