cdc37 is essential for JNK pathway activation and wound closure in Drosophila
- PMID: 31483695
- PMCID: PMC6761768
- DOI: 10.1091/mbc.E18-12-0822
cdc37 is essential for JNK pathway activation and wound closure in Drosophila
Abstract
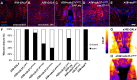
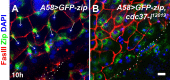
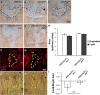
Wound closure in the Drosophila larval epidermis mainly involves nonproliferative, endocyling epithelial cells. Consequently, it is largely mediated by cell growth and migration. We discovered that both cell growth and migration in Drosophila require the cochaperone-encoding gene cdc37. Larvae lacking cdc37 in the epidermis failed to close wounds, and the cells of the epidermis failed to change cell shape and polarize. Likewise, wound-induced cell growth was significantly reduced, and correlated with a reduction in the size of the cell nucleus. The c-Jun N-terminal kinase (JNK) pathway, which is essential for wound closure, was not typically activated in injured cdc37 knockdown larvae. In addition, JNK, Hep, Mkk4, and Tak1 protein levels were reduced, consistent with previous reports showing that Cdc37 is important for the stability of various client kinases. Protein levels of the integrin β subunit and its wound-induced protein expression were also reduced, reflecting the disruption of JNK activation, which is crucial for expression of integrin β during wound closure. These results are consistent with a role of Cdc37 in maintaining the stability of the JNK pathway kinases, thus mediating cell growth and migration during Drosophila wound healing.
Figures

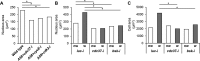
Similar articles
-
Yorkie regulates epidermal wound healing in Drosophila larvae independently of cell proliferation and apoptosis.Dev Biol. 2017 Jul 1;427(1):61-71. doi: 10.1016/j.ydbio.2017.05.006. Epub 2017 May 14. Dev Biol. 2017. PMID: 28514643 Free PMC article.
-
Nonmuscle myosin II localization is regulated by JNK during Drosophila larval wound healing.Biochem Biophys Res Commun. 2010 Mar 19;393(4):656-61. doi: 10.1016/j.bbrc.2010.02.047. Epub 2010 Feb 12. Biochem Biophys Res Commun. 2010. PMID: 20153725
-
Spatiotemporal regulation of cell fusion by JNK and JAK/STAT signaling during Drosophila wound healing.J Cell Sci. 2017 Jun 1;130(11):1917-1928. doi: 10.1242/jcs.187658. Epub 2017 Apr 19. J Cell Sci. 2017. PMID: 28424232
-
Signaling pathways directing the movement and fusion of epithelial sheets: lessons from dorsal closure in Drosophila.Differentiation. 2002 Jun;70(4-5):181-203. doi: 10.1046/j.1432-0436.2002.700408.x. Differentiation. 2002. PMID: 12147138 Review.
-
Regulating cell morphogenesis: the Drosophila Jun N-terminal kinase pathway.Genesis. 2013 Mar;51(3):147-62. doi: 10.1002/dvg.22354. Epub 2012 Nov 20. Genesis. 2013. PMID: 23109363 Review.
Cited by
-
Advanced 3D dynamic culture system with transforming growth factor-β3 enhances production of potent extracellular vesicles with modified protein cargoes via upregulation of TGF-β signaling.J Adv Res. 2023 May;47:57-74. doi: 10.1016/j.jare.2022.09.005. Epub 2022 Sep 18. J Adv Res. 2023. PMID: 36130685 Free PMC article.
-
Deep learning reveals a damage signalling hierarchy that coordinates different cell behaviours driving wound re-epithelialisation.Development. 2024 Sep 15;151(18):dev202943. doi: 10.1242/dev.202943. Epub 2024 Sep 24. Development. 2024. PMID: 39177163 Free PMC article.
-
Inhibition of Cdc37 Ameliorates Arthritis in Collagen-Induced Arthritis Rats by Inhibiting Synoviocyte Proliferation and Migration Through the ERK Pathway.Inflammation. 2023 Jun;46(3):1022-1035. doi: 10.1007/s10753-023-01789-3. Epub 2023 Mar 15. Inflammation. 2023. PMID: 36920636
-
A Drosophila Model to Study Wound-induced Polyploidization.J Vis Exp. 2020 Jun 9;(160):10.3791/61252. doi: 10.3791/61252. J Vis Exp. 2020. PMID: 32597839 Free PMC article.
-
Distinctive features of Zaprionus indianus hemocyte differentiation and function revealed by transcriptomic analysis.Front Immunol. 2023 Dec 21;14:1322381. doi: 10.3389/fimmu.2023.1322381. eCollection 2023. Front Immunol. 2023. PMID: 38187383 Free PMC article.
References
-
- Angel P, Szabowski A, Schorpp-Kistner M. (2001). Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene , 2413–2423. - PubMed
-
- Baek SH, Kwon YC, Lee H, Choe KM. (2010). Rho-family small GTPases are required for cell polarization and directional sensing in Drosophila wound healing. Biochem Biophys Res Commun , 488–492. - PubMed
-
- Bosch M, Serras F, Martin-Blanco E, Baguna J. (2005). JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev Biol , 73–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

