Large gaze shift generation while standing: the role of the vestibular system
- PMID: 31483710
- PMCID: PMC6879955
- DOI: 10.1152/jn.00343.2019
Large gaze shift generation while standing: the role of the vestibular system
Abstract
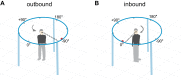
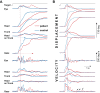
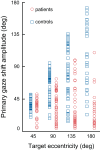
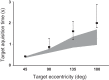
The functional significance of vestibular information for the generation of gaze shifts is controversial and less well established than the vestibular contribution to gaze stability. In this study, we asked seven bilaterally avestibular patients to execute voluntary, whole body pivot turns to visual targets up to 180° while standing. In these conditions, not only are the demands imposed on gaze transfer mechanisms more challenging, but also neck proprioceptive input represents an inadequate source of head-in-space motion information. Patients' body segment was slower and jerky. In the absence of visual feedback, gaze advanced in small steps, closely resembling normal multiple-step gaze-shift patterns, but as a consequence of the slow head motion, target acquisition was delayed. In ~25% of trials, however, patients moved faster but the velocity of prematurely emerging slow-phase compensatory eye movements remained lower than head-in-space velocity due to vestibuloocular failure. During these trials, therefore, gaze advanced toward the target without interruption but, again, taking longer than when normal controls use single-step gaze transfers. That is, even when patients attempted faster gaze shifts, exposing themselves to gaze instability, they acquired distant targets significantly later than controls. Thus, while patients are upright, loss of vestibular information disrupts not only gaze stability but also gaze transfers. The slow and ataxic head and trunk movements introduce significant foveation delays. These deficits explain patients' symptoms during upright activities and show, for the first time, the clinical significance of losing the so-called "anticompensatory" (gaze shifting) function of the vestibuloocular reflex.NEW & NOTEWORTHY Previous studies in sitting avestibular patients concluded that gaze transfers are not substantially compromised. Still, clinicians know that patients are impeded (e.g., looking side to side before crossing a road). We show that during large gaze transfers while standing, vestibularly derived head velocity signals are critical for the mechanisms governing reorientation to distant targets and multisegmental coordination. Our findings go beyond the traditional role of the vestibular system in gaze stability, extending it to gaze transfers, as well.
Keywords: anticompensatory; bilateral vestibular loss; coordination; gaze; multisegmental; turns.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
Fast gaze reorientations by combined movements of the eye, head, trunk and lower extremities.Exp Brain Res. 2015 May;233(5):1639-50. doi: 10.1007/s00221-015-4238-4. Epub 2015 Mar 12. Exp Brain Res. 2015. PMID: 25761968 Free PMC article.
-
Phase-plane analysis of gaze stabilization to high acceleration head thrusts: a continuum across normal subjects and patients with loss of vestibular function.J Neurophysiol. 2004 Apr;91(4):1763-81. doi: 10.1152/jn.00611.2002. Epub 2003 Dec 3. J Neurophysiol. 2004. PMID: 14657187
-
Gaze stabilization during dynamic posturography in normal and vestibulopathic humans.Exp Brain Res. 1998 Sep;122(2):235-46. doi: 10.1007/s002210050511. Exp Brain Res. 1998. PMID: 9776522 Clinical Trial.
-
Gaze stabilisation exercises in vestibular rehabilitation: review of the evidence and recent clinical advances.J Neurol. 2019 Sep;266(Suppl 1):11-18. doi: 10.1007/s00415-019-09459-x. Epub 2019 Aug 5. J Neurol. 2019. PMID: 31385017 Review.
-
Role of vestibular adaptation in vestibular rehabilitation.Otolaryngol Head Neck Surg. 1998 Jul;119(1):49-54. doi: 10.1016/S0194-5998(98)70195-0. Otolaryngol Head Neck Surg. 1998. PMID: 9674514 Review.
Cited by
-
Gaze shift dynamic visual acuity: A functional test of gaze stability that distinguishes unilateral vestibular hypofunction.J Vestib Res. 2021;31(1):23-32. doi: 10.3233/VES-201506. J Vestib Res. 2021. PMID: 33325420 Free PMC article.
-
Voluntary suppression of neck reflexes during passive head-on-trunk rotations: reflex gain control versus proprioceptive feedback.J Neurophysiol. 2022 Jan 1;127(1):161-172. doi: 10.1152/jn.00297.2021. Epub 2021 Dec 15. J Neurophysiol. 2022. PMID: 34907798 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

