Display of malaria transmission-blocking antigens on chimeric duck hepatitis B virus-derived virus-like particles produced in Hansenula polymorpha
- PMID: 31483818
- PMCID: PMC6726142
- DOI: 10.1371/journal.pone.0221394
Display of malaria transmission-blocking antigens on chimeric duck hepatitis B virus-derived virus-like particles produced in Hansenula polymorpha
Abstract
Background: Malaria caused by Plasmodium falciparum is one of the major threats to human health globally. Despite huge efforts in malaria control and eradication, highly effective vaccines are urgently needed, including vaccines that can block malaria transmission. Chimeric virus-like particles (VLP) have emerged as a promising strategy to develop new malaria vaccine candidates.
Methods: We developed yeast cell lines and processes for the expression of malaria transmission-blocking vaccine candidates Pfs25 and Pfs230 as VLP and VLP were analyzed for purity, size, protein incorporation rate and expression of malaria antigens.
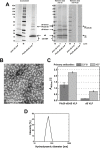
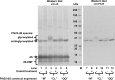
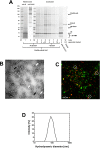
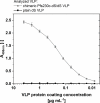
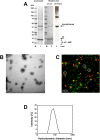
Results: In this study, a novel platform for the display of Plasmodium falciparum antigens on chimeric VLP is presented. Leading transmission-blocking vaccine candidates Pfs25 and Pfs230 were genetically fused to the small surface protein (dS) of the duck hepatitis B virus (DHBV). The resulting fusion proteins were co-expressed in recombinant Hansenula polymorpha (syn. Pichia angusta, Ogataea polymorpha) strains along with the wild-type dS as the VLP scaffold protein. Through this strategy, chimeric VLP containing Pfs25 or the Pfs230-derived fragments Pfs230c or Pfs230D1M were purified. Up to 100 mg chimeric VLP were isolated from 100 g dry cell weight with a maximum protein purity of 90% on the protein level. Expression of the Pfs230D1M construct was more efficient than Pfs230c and enabled VLP with higher purity. VLP showed reactivity with transmission-blocking antibodies and supported the surface display of the malaria antigens on the native VLP.
Conclusion: The incorporation of leading Plasmodium falciparum transmission-blocking antigens into the dS-based VLP scaffold is a promising novel strategy for their display on nano-scaled particles. Competitive processes for efficient production and purification were established in this study.
Conflict of interest statement
The authors VJ, MP, MS, DW and MW are associated with ARTES Biotechnology GmbH which owns the license for the VLP technology: Viral vectors expressing fusion of viral large envelope protein and protein of interest (No. WO2004092387A1). Recombinant proteins and virus-like particles comprising L and S polypeptides of avian hepadnaviridae and methods, nucleic acid constructs, vectors and host cells for producing same (No. WO2008025067A1). Author JM is affiliated with Evonik Technology & Infrastructure GmbH. There are no further patents, products in development or marketed products to declare. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures
References
-
- WHO. World Malaria Report 2017. World Health Organization; 2017. 10.1071/EC12504 - DOI
-
- Malaria Vaccine Funders Group. Malaria Vaccine Technology Roadmap. 2013; 1–9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

