Safety of light emitting diode-red light on human skin: Two randomized controlled trials
- PMID: 31483941
- PMCID: PMC8887049
- DOI: 10.1002/jbio.201960014
Safety of light emitting diode-red light on human skin: Two randomized controlled trials
Abstract
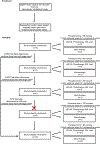
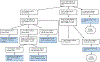
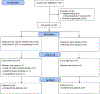
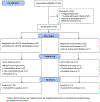
Therapeutic applications of light emitting diode-red light (LED-RL) are expanding, yet data on its clinical effects are lacking. Our goal was to evaluate the safety of high fluence LED-RL (≥160 J/cm2 ). In two phase I, single-blind, dose escalation, randomized controlled trials, healthy subjects received LED-RL or mock irradiation to the forearm thrice weekly for 3 weeks at fluences of 160-640 J/cm2 for all skin types (STARS 1, n = 60) and at 480-640 J/cm2 for non-Hispanic Caucasians (STARS 2, n = 55). The primary outcome was the incidence of adverse events (AEs). The maximum tolerated dose was the highest fluence that did not elicit predefined AEs. Dose-limiting AEs, including blistering and prolonged erythema, occurred at 480 J/cm2 in STARS 1 (n = 1) and 640 J/cm2 in STARS 2 (n = 2). AEs of transient erythema and hyperpigmentation were mild. No serious AEs occurred. We determined that LED-RL is safe up to 320 J/cm2 for skin of color and 480 J/cm2 for non-Hispanic Caucasian individuals. LED-RL may exert differential cutaneous effects depending on race and ethnicity, with darker skin being more photosensitive. These findings may guide future studies to evaluate the efficacy of LED-RL for the treatment of various diseases.
Keywords: low-level light therapy; phototherapy; randomized controlled trial; skin pigmentation.
© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures