Mechanisms and Implications of Metabolic Heterogeneity in Cancer
- PMID: 31484055
- PMCID: PMC6730674
- DOI: 10.1016/j.cmet.2019.08.013
Mechanisms and Implications of Metabolic Heterogeneity in Cancer
Abstract
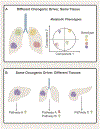
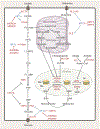
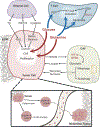
Tumors display reprogrammed metabolic activities that promote cancer progression. We currently possess a limited understanding of the processes governing tumor metabolism in vivo and of the most efficient approaches to identify metabolic vulnerabilities susceptible to therapeutic targeting. While much of the literature focuses on stereotyped, cell-autonomous pathways like glycolysis, recent work emphasizes heterogeneity and flexibility of metabolism between tumors and even within distinct regions of solid tumors. Metabolic heterogeneity is important because it influences therapeutic vulnerabilities and may predict clinical outcomes. This Review describes current concepts about metabolic regulation in tumors, focusing on processes intrinsic to cancer cells and on factors imposed upon cancer cells by the tumor microenvironment. We discuss experimental approaches to identify subtype-selective metabolic vulnerabilities in preclinical cancer models. Finally, we describe efforts to characterize metabolism in primary human tumors, which should produce new insights into metabolic heterogeneity in the context of clinically relevant microenvironments.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures

References
-
- Allis CD, and Jenuwein T (2016). The molecular hallmarks of epigenetic control. Nature Reviews Genetics 17, 487. - PubMed
-
- Bonuccelli G, Whitaker-Menezes D, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, Witkiewicz AK, Vander Heiden MG, Migneco G, Chiavarina B, et al. (2010). The reverse Warburg effect: glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell cycle (Georgetown, Tex.) 9, 1960–1971. - PubMed
-
- Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et al. (2016). LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell metabolism 24, 657–671. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

