The Mechanisms Involved in Morphine Addiction: An Overview
- PMID: 31484312
- PMCID: PMC6747116
- DOI: 10.3390/ijms20174302
The Mechanisms Involved in Morphine Addiction: An Overview
Abstract
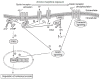
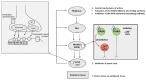
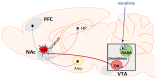
Opioid use disorder is classified as a chronic recurrent disease of the central nervous system (CNS) which leads to personality disorders, co-morbidities and premature death. It develops as a result of long-term administration of various abused substances, along with morphine. The pharmacological action of morphine is associated with its stimulation of opioid receptors. Opioid receptors are a group of G protein-coupled receptors and activation of these receptors by ligands induces significant molecular changes inside the cell, such as an inhibition of adenylate cyclase activity, activation of potassium channels and reductions of calcium conductance. Recent data indicate that other signalling pathways also may be involved in morphine activity. Among these are phospholipase C, mitogen-activated kinases (MAP kinases) or β-arrestin. The present review focuses on major mechanisms which currently are considered as essential in morphine activity and dependence and may be important for further studies.
Keywords: adenylate cyclase activity; mesolimbic system; mitogen-activated kinases (MAP kinases); morphine tolerance and withdrawal signs; opioid receptors; β-arrestin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence.Nature. 2000 Dec 7;408(6813):720-3. doi: 10.1038/35047086. Nature. 2000. PMID: 11130073
-
Morphine dependence in human neuroblastoma SH-SY5Y cells is associated with adaptive changes in both the quantity and functional interaction of PGE1 receptors and stimulatory G proteins.Brain Res. 1996 Jan 29;707(2):235-44. doi: 10.1016/0006-8993(95)01265-6. Brain Res. 1996. PMID: 8919301
-
Complex formation between the vasopressin 1b receptor, β-arrestin-2, and the μ-opioid receptor underlies morphine tolerance.Nat Neurosci. 2018 Jun;21(6):820-833. doi: 10.1038/s41593-018-0144-y. Epub 2018 Apr 30. Nat Neurosci. 2018. PMID: 29713080
-
Reflections on: "A general role for adaptations in G-Proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function".Brain Res. 2016 Aug 15;1645:71-4. doi: 10.1016/j.brainres.2015.12.039. Epub 2015 Dec 29. Brain Res. 2016. PMID: 26740398 Free PMC article. Review.
-
Chronic morphine-induced plasticity among signalling molecules.Novartis Found Symp. 2004;261:167-76; discussion 176-80, 191-3. Novartis Found Symp. 2004. PMID: 15469050 Review.
Cited by
-
Experimental and real-world evidence supporting the computational repurposing of bumetanide for APOE4-related Alzheimer's disease.Nat Aging. 2021 Oct;1(10):932-947. doi: 10.1038/s43587-021-00122-7. Epub 2021 Oct 11. Nat Aging. 2021. PMID: 36172600 Free PMC article.
-
Involvement of Basolateral Amygdala Dopamine D1 Receptors in the Acquisition and Expression of Morphine-Induced Place Preference in Rats.Adv Biomed Res. 2022 Jan 31;11:8. doi: 10.4103/abr.abr_284_21. eCollection 2022. Adv Biomed Res. 2022. PMID: 35284350 Free PMC article.
-
Role of the Orexinergic System Within the Ventral Tegmental Area in the Development of Sensitization to Morphine Induced by Lateral Hypothalamus Stimulation.Basic Clin Neurosci. 2022 Jan-Feb;13(1):97-106. doi: 10.32598/bcn.2021.2946.1. Epub 2022 Jan 1. Basic Clin Neurosci. 2022. PMID: 36589022 Free PMC article.
-
Novel N,Cl-doped deep eutectic solvents-based carbon dots as a selective fluorescent probe for determination of morphine in food.RSC Adv. 2021 May 7;11(27):16805-16813. doi: 10.1039/d1ra00886b. eCollection 2021 Apr 30. RSC Adv. 2021. PMID: 35479173 Free PMC article.
-
Opioids and Vitamin C: Known Interactions and Potential for Redox-Signaling Crosstalk.Antioxidants (Basel). 2022 Jun 27;11(7):1267. doi: 10.3390/antiox11071267. Antioxidants (Basel). 2022. PMID: 35883757 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

