Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress
- PMID: 31484323
- PMCID: PMC6769627
- DOI: 10.3390/nu11092062
Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress
Abstract
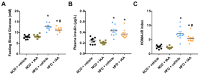
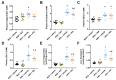
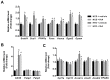
Recent evidences have linked indole-3-acetic acid (IAA), a gut microbiota-derived metabolite from dietary tryptophan, with the resistance to liver diseases. However, data supporting IAA-mediated protection against nonalcoholic fatty liver disease (NAFLD) from an in vivo study is lacking. In this study, we assessed the role of IAA in attenuating high-fat diet (HFD)-induced NAFLD in male C57BL/6 mice. Administration of IAA (50 mg/kg body weight) by intraperitoneal injection was found to alleviate HFD-induced elevation in fasting blood glucose and homeostasis model assessment of insulin resistance (HOMA-IR) index as well as plasma total cholesterol, low-density lipoprotein cholesterol (LDL-C), and glutamic-pyruvic transaminase (GPT) activity. Histological examination further presented the protective effect of IAA on liver damage induced by HFD feeding. HFD-induced an increase in liver total triglycerides and cholesterol, together with the upregulation of genes related to lipogenesis including sterol regulatory element binding-protein 1 (Srebf1), steraroyl coenzyme decarboxylase 1 (Scd1), peroxisome proliferator-activated receptor gamma (PPARγ), acetyl-CoA carboxylase 1 (Acaca), and glycerol-3-phosphate acyltransferase, mitochondrial (Gpam), which were mitigated by IAA treatment. The results of reactive oxygen species (ROS) and malonaldehyde (MDA) level along with superoxide dismutase (SOD) activity and glutathione (GSH) content in liver tissue evidenced the protection of IAA against HFD-induced oxidative stress. Additionally, IAA attenuated the inflammatory response of liver in mice exposed to HFD as shown by the reduction in the F4/80-positive macrophage infiltration and the expression of monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor-α (TNF-α). In conclusion, our findings uncover that IAA alleviates HFD-induced hepatotoxicity in mice, which proves to be associated with the amelioration in insulin resistance, lipid metabolism, and oxidative and inflammatory stress.
Keywords: NAFLD; indole-3-acetic acid; inflammation; lipid metabolism; oxidative stress; steatosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Rau M., Schilling A.K., Meertens J., Hering I., Weiss J., Jurowich C., Kudlich T., Hermanns H.M., Bantel H., Beyersdorf N., et al. Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J. Immunol. 2016;196:97–105. doi: 10.4049/jimmunol.1501175. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

