Long-Term Growth in Phenylketonuria: A Systematic Review and Meta-Analysis
- PMID: 31484352
- PMCID: PMC6769966
- DOI: 10.3390/nu11092070
Long-Term Growth in Phenylketonuria: A Systematic Review and Meta-Analysis
Abstract
There is an ongoing debate regarding the impact of phenylketonuria (PKU) and its treatment on growth. To date, evidence from studies is inconsistent, and data on the whole developmental period is limited. The primary aim of this systematic review was to investigate the effects of a phenylalanine (Phe)-restricted diet on long-term growth in patients with PKU. Four electronic databases were searched for articles published until September 2018. A total of 887 results were found, but only 13 articles met eligibility criteria. Only three studies had an adequate methodology for meta-analysis. Although the results indicate normal growth at birth and during infancy, children with PKU were significantly shorter and had lower weight for age than reference populations during the first four years of life. Impaired linear growth was observed until the end of adolescence in PKU. In contrast, growth impairment was not reported in patients with mild hyperphenylalaninemia, not requiring dietary restriction. Current evidence indicates that even with advances in dietary treatments, "optimal" growth outcomes are not attained in PKU. The majority of studies include children born before 1990s, so further research is needed to show the effects of recent dietary practices on growth in PKU.
Keywords: anthropometrics; growth; height; hyperphenylalaninemia; phenylketonuria; weight; z-scores.
Conflict of interest statement
Alex Pinto has received an educational grant from Cambrooke Therapeutics and grants from Vitaflo, Nutricia, Merck Serono, and Biomarin to attend scientific meetings. Hulya Gokmen-Ozel is a member of the European Nutrition Expert Panel (Biomarin) and is on the Scientific Advisory Board(s) of Nutricia Metabolics Turkey. Júlio César Rocha is member of the European Nutrition Expert Panel (Biomarin), member of an Advisory Board for Applied Pharma Research, and has received fees from Merck Serono, Vitaflo, Nutricia, and Cambrooke. Esther van Dam and Kirsten Ahring are members of the European Nutrition Expert Panel (Biomarin). A. Bélanger-Quintana has received honoraria as a speaker from Nutricia, Vitaflo International, Merck Serono, and Recordati and is a member of the European Nutrition Expert Panel (Merck Serono international, Biomarin), the Sapropterin Advisory Board (Merck Serono international, Biomarin), and KAMPER Advisory Board (Merck Serono International, Biomarin). Katharina Dokoupil has received honoraria from Nutricia, Vitaflo, Merck Serono, and Schär, grants to attend the SSIEM Annual Symposium from Nutricia Metabolics, and is a member of the European Nutrition Expert Panel (Biomarin), member of a Nutricia Advisory Board, and member of a Nestlé Advisory Board. Anita MacDonald has received research funding and honoraria from Nutricia, Vitaflo International, and Merck Serono. She is a member of the European Nutrition Expert Panel (Biomarin), member of Sapropterin Advisory Board (Biomarin), member of the Advisory Board entitled ELEMENT (Danone-Nutricia), and member of an Advisory Board for Arla and Applied Pharma Research. Fatma Ilgaz and Erdem Karabulut declare no conflict of interest.
Figures
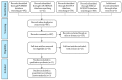

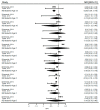
References
-
- van Wegberg A.M.J., MacDonald A., Ahring K., Bélanger-Quintana A., Blau N., Bosch A.M., Burlina A., Campistol J., Feillet F., Giżewska M., et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017;12:162. doi: 10.1186/s13023-017-0685-2. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

