Alcohol-Supported Cu-Mediated 18F-Fluorination of Iodonium Salts under "Minimalist" Conditions
- PMID: 31484375
- PMCID: PMC6749259
- DOI: 10.3390/molecules24173197
Alcohol-Supported Cu-Mediated 18F-Fluorination of Iodonium Salts under "Minimalist" Conditions
Abstract
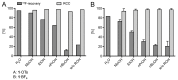
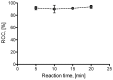
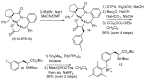
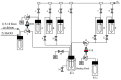
In the era of personalized precision medicine, positron emission tomography (PET) and related hybrid methods like PET/CT and PET/MRI gain recognition as indispensable tools of clinical diagnostics. A broader implementation of these imaging modalities in clinical routine is closely dependent on the increased availability of established and emerging PET-tracers, which in turn could be accessible by the development of simple, reliable, and efficient radiolabeling procedures. A further requirement is a cGMP production of imaging probes in automated synthesis modules. Herein, a novel protocol for the efficient preparation of 18F-labeled aromatics via Cu-mediated radiofluorination of (aryl)(mesityl)iodonium salts without the need of evaporation steps is described. Labeled aromatics were prepared in high radiochemical yields simply by heating of iodonium [18F]fluorides with the Cu-mediator in methanolic DMF. The iodonium [18F]fluorides were prepared by direct elution of 18F- from an anion exchange resin with solutions of the corresponding precursors in MeOH/DMF. The practicality of the novel method was confirmed by the racemization-free production of radiolabeled fluorophenylalanines, including hitherto unknown 3-[18F]FPhe, in 22-69% isolated radiochemical yields as well as its direct implementation into a remote-controlled synthesis unit.
Keywords: 18F; copper-mediated; iodonium salts; positron emission tomography; radiolabeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

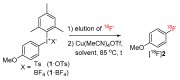


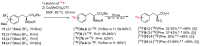

References
-
- Hoover A.J., Lazari M., Ren H., Narayanam M.K., Murphy J.M., van Dam R.M., Hooker J.M., Ritter T. A Transmetalation Reaction Enables the Synthesis of [18F]5-Fluorouracil from [18F]Fluoride for Human PET Imaging. Organometallics. 2016;35:1008–1014. doi: 10.1021/acs.organomet.6b00059. - DOI - PMC - PubMed
-
- Zlatopolskiy B.D., Zischler J., Urusova E.A., Endepols H., Kordys E., Frauendorf H., Mottaghy F.M., Neumaier B. A Practical One-Pot Synthesis of Positron Emission Tomography (PET) Tracers via Nickel-Mediated Radiofluorination. ChemistryOpen. 2015;4:457–462. doi: 10.1002/open.201500056. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

