Asymmetric and Reduced Xanthene Fluorophores: Synthesis, Photochemical Properties, and Application to Activatable Fluorescent Probes for Detection of Nitroreductase
- PMID: 31484448
- PMCID: PMC6749439
- DOI: 10.3390/molecules24173206
Asymmetric and Reduced Xanthene Fluorophores: Synthesis, Photochemical Properties, and Application to Activatable Fluorescent Probes for Detection of Nitroreductase
Abstract
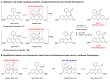
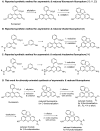
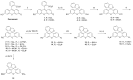
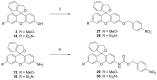
Xanthene fluorophores, including fluorescein, rhodol, and rhodamines, are representative classes of fluorescent probes that have been applied in the detection and visualization of biomolecules. "Turn on" activatable fluorescent probes, that can be turned on in response to enzymatic reactions, have been developed and prepared to reduce the high background signal of "always-on" fluorescent probes. However, the development of activity-based fluorescent probes for biological applications, using simple xanthene dyes, is hampered by their inefficient synthetic methods and the difficulty of chemical modifications. We have, thus, developed a highly efficient, versatile synthetic route to developing chemically more stable reduced xanthene fluorophores, based on fluorescein, rhodol, and rhodamine via continuous Pd-catalyzed cross-coupling. Their fluorescent nature was evaluated by monitoring fluorescence with variation in the concentration, pH, and solvent. As an application to activatable fluorescent probe, nitroreductase (NTR)-responsive fluorescent probes were also developed using the reduced xanthene fluorophores, and their fluorogenic properties were evaluated.
Keywords: activatable fluorescent probe; fluorescence; nitroreductase; reduced rhodafluor; xanthene fluorophore.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Silicon-substituted Xanthene Dyes and Their Unique Photophysical Properties for Fluorescent Probes.Chem Asian J. 2017 Jul 4;12(13):1435-1446. doi: 10.1002/asia.201700385. Epub 2017 Jun 5. Chem Asian J. 2017. PMID: 28452155 Review.
-
Advances in modifying fluorescein and rhodamine fluorophores as fluorescent chemosensors.Chem Commun (Camb). 2013 Jan 18;49(5):429-47. doi: 10.1039/c2cc35997a. Chem Commun (Camb). 2013. PMID: 23164947
-
Cell-Permeable Fluorogenic Probes for Identification and Imaging Nitroreductases in Live Bacterial Cells.J Org Chem. 2019 Feb 1;84(3):1299-1309. doi: 10.1021/acs.joc.8b02746. Epub 2019 Jan 11. J Org Chem. 2019. PMID: 30589544
-
Fluorogenic and Mitochondria-Localizable Probe Enables Selective Labeling and Imaging of Nitroreductase.Anal Chem. 2022 May 24;94(20):7272-7277. doi: 10.1021/acs.analchem.2c00512. Epub 2022 May 12. Anal Chem. 2022. PMID: 35549110
-
[Development of Novel Dark Quenchers and Their Application to Imaging Probes].Yakugaku Zasshi. 2019;139(2):277-283. doi: 10.1248/yakushi.18-00174-3. Yakugaku Zasshi. 2019. PMID: 30713240 Review. Japanese.
Cited by
-
A coumarin derivative-Cu2+ complex-based fluorescent chemosensor for detection of biothiols.RSC Adv. 2020 Oct 1;10(60):36265-36274. doi: 10.1039/d0ra05651k. eCollection 2020 Oct 1. RSC Adv. 2020. PMID: 35517943 Free PMC article.
-
Rapid differentiation between bacterial infections and cancer using a near-infrared fluorogenic probe.Chem Sci. 2020 Feb 26;11(12):3141-3145. doi: 10.1039/d0sc00508h. Chem Sci. 2020. PMID: 34122818 Free PMC article.
-
Coumarin-Based Dual Chemosensor for Colorimetric and Fluorescent Detection of Cu2+ in Water Media.ACS Omega. 2020 Aug 13;5(33):21241-21249. doi: 10.1021/acsomega.0c03097. eCollection 2020 Aug 25. ACS Omega. 2020. PMID: 32875260 Free PMC article.
-
Oxyethylated Fluoresceine-(thia)calix[4]arene Conjugates: Synthesis and Visible-Light Photoredox Catalysis in Water-Organic Media.Molecules. 2022 Dec 28;28(1):261. doi: 10.3390/molecules28010261. Molecules. 2022. PMID: 36615457 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

