A multicenter RCT of noninvasive ventilation in pneumonia-induced early mild acute respiratory distress syndrome
- PMID: 31484582
- PMCID: PMC6727327
- DOI: 10.1186/s13054-019-2575-6
A multicenter RCT of noninvasive ventilation in pneumonia-induced early mild acute respiratory distress syndrome
Abstract
Rationale: Our pilot study suggested that noninvasive ventilation (NIV) reduced the need for intubation compared with conventional administration of oxygen on patients with "early" stage of mild acute respiratory distress syndrome (ARDS, PaO2/FIO2 between 200 and 300).
Objectives: To evaluate whether early NIV can reduce the need for invasive ventilation in patients with pneumonia-induced early mild ARDS.
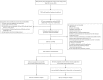
Methods: Prospective, multicenter, randomized controlled trial (RCT) of NIV compared with conventional administration of oxygen through a Venturi mask. Primary outcome included the numbers of patients who met the intubation criteria.
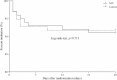
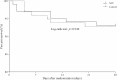
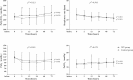
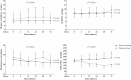
Results: Two hundred subjects were randomized to NIV (n = 102) or control (n = 98) groups from 21 centers. Baseline characteristics were similar in the two groups. In the NIV group, PaO2/FIO2 became significantly higher than in the control group at 2 h after randomization and remained stable for the first 72 h. NIV did not decrease the proportion of patients requiring intubation than in the control group (11/102 vs. 9/98, 10.8% vs. 9.2%, p = 0.706). The ICU mortality was similar in the two groups (7/102 vs. 7/98, 4.9% vs. 3.1%, p = 0.721). Multivariate analysis showed minute ventilation greater than 11 L/min at 48 h was the independent risk factor for NIV failure (OR, 1.176 [95% CI, 1.005-1.379], p = 0.043).
Conclusions: Treatment with NIV did not reduce the need for intubation among patients with pneumonia-induced early mild ARDS, despite the improved PaO2/FIO2 observed with NIV compared with standard oxygen therapy. High minute ventilation may predict NIV failure.
Trial registration: NCT01581229 . Registered 19 April 2012.
Keywords: Acute respiratory distress syndrome (ARDS); Noninvasive ventilation (NIV); Pneumonia.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Comment in
-
Noninvasive ventilation in patients with acute respiratory distress syndrome.Crit Care. 2019 Nov 15;23(1):358. doi: 10.1186/s13054-019-2666-4. Crit Care. 2019. PMID: 31730002 Free PMC article. No abstract available.
References
-
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. - DOI - PubMed
-
- Antonelli M, Conti G, Esquinas A, Montini L, Maggiore SM, Bello G, Rocco M, Maviglia R, Pennisi MA, Gonzalez-Diaz G, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18–25. doi: 10.1097/01.CCM.0000251821.44259.F3. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

