Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma
- PMID: 31484647
- PMCID: PMC6888142
- DOI: 10.1182/blood.2019000241
Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma
Abstract
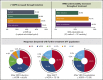
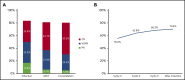
Achieving and maintaining a high-quality response is the treatment goal for patients with newly diagnosed multiple myeloma (NDMM). The phase 3 PETHEMA/GEM2012 study, in 458 patients aged ≤65 years with NDMM, is evaluating bortezomib (subcutaneous) + lenalidomide + dexamethasone (VRD) for 6 cycles followed by autologous stem cell transplant (ASCT) conditioned with IV busulfan + melphalan vs melphalan and posttransplant consolidation with 2 cycles of VRD. We present grouped response analysis of induction, transplant, and consolidation. Responses deepened over time; in patients who initiated cycle 6 of induction (n = 426), the rates of a very good partial response or better were 55.6% by cycle 3, 63.8% by cycle 4, 68.3% by cycle 5, and 70.4% after induction. The complete response rate of 33.4% after induction in the intent-to-treat (ITT) population, which was similar in the 92 patients with high-risk cytogenetics (34.8%), also deepened with further treatment (44.1% after ASCT and 50.2% after consolidation). Rates of undetectable minimal residual disease (median 3 × 10-6 sensitivity) in the ITT population also increased from induction (28.8%) to transplant (42.1%) and consolidation (45.2%). The most common grade ≥3 treatment-emergent adverse events during induction were neutropenia (12.9%) and infection (9.2%). Grade ≥2 peripheral neuropathy (grouped term) during induction was 17.0%, with a low frequency of grade 3 (3.7%) and grade 4 (0.2%) events. VRD is an effective and well-tolerated regimen for induction in NDMM with deepening response throughout induction and over the course of treatment. This trial was registered at www.clinicaltrials.gov as #NCT01916252 and EudraCT as #2012-005683-10.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: L.R. reports honoraria from Janssen, Celgene, Amgen, and Takeda. A.O. reports consultancy for and membership on board of directors, advisory committees, or speakers bureaus with Amgen, Celgene, Takeda, and Janssen. R.R. reports consultancy with Amgen, Celgene, Janssen, and Takeda. A.S. reports honoraria from Bristol-Myers Squibb, Takeda, Sanofi, Merck, and Roche; consultancy with BMS, Takeda, and Merck; and membership on speakers bureaus with Takeda. J. M. Moraleda reports membership on advisory committees with Gilead, Celgene, and Takeda. I.J. reports honoraria from Janssen, Celgene, Roche, Novartis, Amgen, and Takeda. F.d.A. reports honoraria from and consultancy with Celgene, Janssen, and Amgen. J. M. Martí reports membership on speakers bureau with Celgene and reimbursement of travel, accommodations, and/or expenses by Janssen and Celgene. J.M.A. reports honoraria from Celgene, Amgen, Janssen, Novartis, Takeda, and Roche. L.F.C. reports honoraria for lectures from and membership on advisory boards with Celgene, Janssen, Roche, Novartis, Bristol-Myers Squibb, Amgen, Takeda, Pfizer, Incyte, and AbbVie. B.P. reports honoraria for lectures from and membership on advisory boards with Amgen, Bristol-Myers Squibb, Celgene, Janssen, Merck, Novartis, Roche, and Sanofi; unrestricted grants from Celgene, EngMab, Sanofi, and Takeda; and consultancy for Celgene, Janssen, and Sanofi. M.-V.M. reports consultancy for, honoraria from, and membership on board of directors or advisory committees with Janssen, Celgene, GSK, Takeda, and Amgen. J.F.S.M. reports consultancy for Bristol-Myers Squibb, Celgene, Novartis, Takeda, Amgen, MSD, Janssen, and Sanofi and membership on board of directors or advisory committees with Takeda. J.-J.L. reports honoraria from and membership on board of directors or advisory committees with Takeda, Amgen, Celgene, and Janssen. J. Bladé reports honoraria from Celgene, Amgen, and Janssen. The remaining authors declare no competing financial interests.
Figures
References
-
- Lahuerta JJ, Mateos MV, Martínez-López J, et al. . Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26(35):5775-5782. - PubMed
-
- Harousseau JL, Avet-Loiseau H, Attal M, et al. . Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: long-term analysis of the IFM 99-02 and 99-04 trials. J Clin Oncol. 2009;27(34):5720-5726. - PubMed
-
- van de Velde HJ, Liu X, Chen G, Cakana A, Deraedt W, Bayssas M. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92(10):1399-1406. - PubMed
-
- Martinez-Lopez J, Blade J, Mateos MV, et al. . Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood. 2011;118(3):529-534. - PubMed
-
- D’Souza A, Fretham C Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2018. https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/Docum.... Accessed 16 September 2019.