Deoxycytidine Release from Pancreatic Stellate Cells Promotes Gemcitabine Resistance
- PMID: 31484670
- PMCID: PMC7357734
- DOI: 10.1158/0008-5472.CAN-19-0960
Deoxycytidine Release from Pancreatic Stellate Cells Promotes Gemcitabine Resistance
Abstract
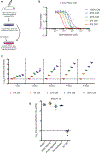
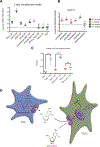
Pancreatic ductal adenocarcinoma (PDAC) is a leading cause of cancer deaths in the United States. The deoxynucleoside analogue gemcitabine is among the most effective therapies to treat PDAC, however, nearly all patients treated with gemcitabine either fail to respond or rapidly develop resistance. One hallmark of PDAC is a striking accumulation of stromal tissue surrounding the tumor, and this accumulation of stroma can contribute to therapy resistance. To better understand how stroma limits response to therapy, we investigated cell-extrinsic mechanisms of resistance to gemcitabine. Conditioned media from pancreatic stellate cells (PSC), as well as from other fibroblasts, protected PDAC cells from gemcitabine toxicity. The protective effect of PSC-conditioned media was mediated by secretion of deoxycytidine, but not other deoxynucleosides, through equilibrative nucleoside transporters. Deoxycytidine inhibited the processing of gemcitabine in PDAC cells, thus reducing the effect of gemcitabine and other nucleoside analogues on cancer cells. These results suggest that reducing deoxycytidine production in PSCs may increase the efficacy of nucleoside analog therapies. SIGNIFICANCE: This study provides important new insight into mechanisms that contribute to gemcitabine resistance in PDAC and suggests new avenues for improving gemcitabine efficacy.
©2019 American Association for Cancer Research.
Figures


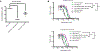
References
-
- Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. , Pancreatic cancer. Nat. Rev. Dis. Prim 2, 1–23 (2016). - PubMed
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM, Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 74, 2913–2921 (2014). - PubMed
-
- Institute NC, Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969–2015). DCCPS, Surveill. Res. Progr (2017), (available at https://seer.cancer.gov).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

