OsCASP1 Is Required for Casparian Strip Formation at Endodermal Cells of Rice Roots for Selective Uptake of Mineral Elements
- PMID: 31484684
- PMCID: PMC6881135
- DOI: 10.1105/tpc.19.00296
OsCASP1 Is Required for Casparian Strip Formation at Endodermal Cells of Rice Roots for Selective Uptake of Mineral Elements
Abstract
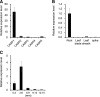
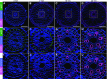
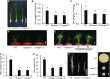
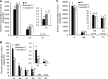
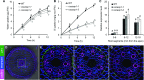
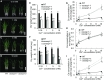
In response to diverse environmental conditions, rice (Oryza sativa) roots have developed one Casparian strip (CS) at the exodermis and one CS at the endodermis. Here, we functionally characterized OsCASP1 (Casparian strip domain protein 1) in rice. OsCASP1 was mainly expressed in the root elongation zone, and the protein encoded was first localized to all sides of the plasma membrane of endodermal cells without CS, followed by the middle of the anticlinal side of endodermal cells with CS. Knockout of OsCASP1 resulted in a defect of CS formation at the endodermis and decreased growth under both soil and hydroponic conditions. Mineral analysis showed that the oscasp1 mutants accumulated more Ca, but less Mn, Zn, Fe, Cd, and As in the shoots compared with the wild type. The growth inhibition of the mutants was further aggravated by high Ca in growth medium. The polar localization of the Si transporter Low Si 1 at the distal side of the endodermis was not altered in the mutant, but the protein abundance was decreased, resulting in a substantial reduction in silicon uptake. These results indicated that OsCASP1 is required for CS formation at the endodermis and that the CS in rice plays an important role in root selective uptake of mineral elements, especially Ca and Si.
© 2019 American Society of Plant Biologists. All rights reserved.
Figures


References
-
- Alassimone J., Fujita S., Doblas V.G., van Dop M., Barberon M., Kalmbach L., Vermeer J.E., Rojas-Murcia N., Santuari L., Hardtke C.S., Geldner N. (2016). Polarly localized kinase SGN1 is required for Casparian strip integrity and positioning. Nat. Plants 2: 16113. - PubMed
-
- Attia H., Arnaud N., Karray N., Lachaal M. (2008). Long-term effects of mild salt stress on growth, ion accumulation and superoxide dismutase expression of Arabidopsis rosette leaves. Physiol. Plant. 132: 293–305. - PubMed
-
- Barberon M., Vermeer J.E., De Bellis D., Wang P., Naseer S., Andersen T.G., Humbel B.M., Nawrath C., Takano J., Salt D.E., Geldner N. (2016). Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164: 447–459. - PubMed
-
- Barbosa I., Rojas-Murcia N., Geldner N. (2019). The Casparian strip-one ring to bring cell biology to lignification? Curr. Opin. Biotechnol. 56: 121–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

