Host Cytoskeleton Remodeling throughout the Blood Stages of Plasmodium falciparum
- PMID: 31484690
- PMCID: PMC6759665
- DOI: 10.1128/MMBR.00013-19
Host Cytoskeleton Remodeling throughout the Blood Stages of Plasmodium falciparum
Abstract
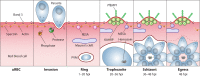
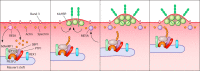
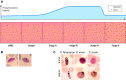
The asexual intraerythrocytic development of Plasmodium falciparum, causing the most severe form of human malaria, is marked by extensive host cell remodeling. Throughout the processes of invasion, intracellular development, and egress, the erythrocyte membrane skeleton is remodeled by the parasite as required for each specific developmental stage. The remodeling is facilitated by a plethora of exported parasite proteins, and the erythrocyte membrane skeleton is the interface of most of the observed interactions between the parasite and host cell proteins. Host cell remodeling has been extensively described and there is a vast body of information on protein export or the description of parasite-induced structures such as Maurer's clefts or knobs on the host cell surface. Here we specifically review the molecular level of each host cell-remodeling step at each stage of the intraerythrocytic development of P. falciparum We describe key events, such as invasion, knob formation, and egress, and identify the interactions between exported parasite proteins and the host cell cytoskeleton. We discuss each remodeling step with respect to time and specific requirement of the developing parasite to explain host cell remodeling in a stage-specific manner. Thus, we highlight the interaction with the host membrane skeleton as a key event in parasite survival.
Keywords: cytoskeleton; erythrocytes; gametocytes; malaria; remodeling.
Copyright © 2019 American Society for Microbiology.
Figures



References
-
- WHO. 2016. World malaria report 2016. WHO, Geneva, Switzerland.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

