Pasteurella multocida: Genotypes and Genomics
- PMID: 31484691
- PMCID: PMC6759666
- DOI: 10.1128/MMBR.00014-19
Pasteurella multocida: Genotypes and Genomics
Abstract
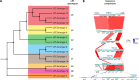
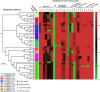
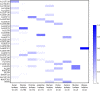
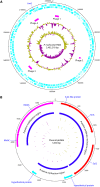
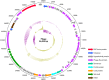
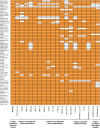
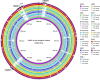
Pasteurella multocida is a highly versatile pathogen capable of causing infections in a wide range of domestic and wild animals as well as in humans and nonhuman primates. Despite over 135 years of research, the molecular basis for the myriad manifestations of P. multocida pathogenesis and the determinants of P. multocida phylogeny remain poorly defined. The current availability of multiple P. multocida genome sequences now makes it possible to delve into the underlying genetic mechanisms of P. multocida fitness and virulence. Using whole-genome sequences, the genotypes, including the capsular genotypes, lipopolysaccharide (LPS) genotypes, and multilocus sequence types, as well as virulence factor-encoding genes of P. multocida isolates from different clinical presentations can be characterized rapidly and accurately. Putative genetic factors that contribute to virulence, fitness, host specificity, and disease predilection can also be identified through comparative genome analysis of different P. multocida isolates. However, although some knowledge about genotypes, fitness, and pathogenesis has been gained from the recent whole-genome sequencing and comparative analysis studies of P. multocida, there is still a long way to go before we fully understand the pathogenic mechanisms of this important zoonotic pathogen. The quality of several available genome sequences is low, as they are assemblies with relatively low coverage, and genomes of P. multocida isolates from some uncommon host species are still limited or lacking. Here, we review recent advances, as well as continuing knowledge gaps, in our understanding of determinants contributing to virulence, fitness, host specificity, disease predilection, and phylogeny of P. multocida.
Keywords: Pasteurella multocida; comparative genomic analysis; disease predilection; fitness; host specificity; phylogeny; virulence; whole-genome sequencing.
Copyright © 2019 American Society for Microbiology.
Figures






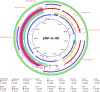
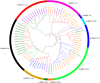
References
-
- Pasteur L. 1880. De l’attenuation du virus du cholera des poules. C R Acad Sci 91:673–680.
-
- Boyce JD, Harper M, Wilkie IW, Adler B. 2010. Pasteurella, p 325–346. In Gyles CL, Prescott JF, Songer JG, Thoen CO (ed), Pathogenesis of bacterial infections in animals, 4th ed Wiley-Blackwell, Ames, IA.
-
- Mutters R, Ihm P, Pohl S, Frederiksen W, Mannheim W. 1985. Reclassification of the genus Pasteurella Trevisan 1887 on the basis of deoxyribonucliec acid homology, with proposals for the new species Pasteurella dagmatis, Pasteurella canis, Pasteurella stomatis, Pasteurella anatis, and Pasteurella langaa. Int J Syst Bacteriol 35:309–322. doi:10.1099/00207713-35-3-309. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

