Onset of clinical and MRI efficacy of ocrelizumab in relapsing multiple sclerosis
- PMID: 31484710
- PMCID: PMC6946481
- DOI: 10.1212/WNL.0000000000008189
Onset of clinical and MRI efficacy of ocrelizumab in relapsing multiple sclerosis
Abstract
Objective: To assess the onset of ocrelizumab efficacy on brain MRI measures of disease activity in the phase II study in relapsing-remitting multiple sclerosis (RRMS), and relapse rate in the pooled phase III studies in relapsing multiple sclerosis (RMS).
Methods: Brain MRI activity was determined in the phase II trial at monthly intervals in patients with RRMS receiving placebo, ocrelizumab (600 mg), or intramuscular interferon (IFN) β-1a (30 μg). Annualized relapse rate (ARR; over various epochs) and time to first relapse were analyzed in the pooled population of the phase III OPERA (A Study of Ocrelizumab in Comparison With Interferon Beta-1a [Rebif] in Participants With Relapsing Multiple Sclerosis) I and OPERA II trials in patients with RMS receiving ocrelizumab (600 mg) or subcutaneous IFN-β-1a (44 μg).
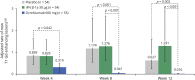
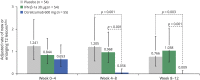
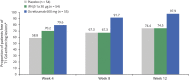
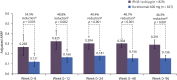
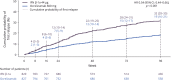
Results: In patients with RRMS, ocrelizumab reduced the number of new T1 gadolinium-enhancing lesions by week 4 vs placebo (p = 0.042) and by week 8 vs intramuscular IFN-β-1a (p < 0.001). Ocrelizumab also reduced the number of new or enlarging T2 lesions appearing between weeks 4 and 8 vs both placebo and IFN-β-1a (both p < 0.001). In patients with RMS, ocrelizumab significantly reduced ARR (p = 0.005) and the probability of time to first protocol-defined relapse (p = 0.014) vs subcutaneous IFN-β-1a within the first 8 weeks.
Conclusion: Epoch analysis of MRI-measured lesion activity in the phase II study and relapse rate in the phase III studies consistently revealed a rapid suppression of acute MRI and clinical disease activity following treatment initiation with ocrelizumab in patients with RRMS and RMS, respectively.
Classification of evidence: This study provides Class II evidence that for patients with RRMS and RMS, ocrelizumab suppressed MRI activity within 4 weeks and clinical disease activity within 8 weeks.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
Similar articles
-
Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis.N Engl J Med. 2017 Jan 19;376(3):221-234. doi: 10.1056/NEJMoa1601277. Epub 2016 Dec 21. N Engl J Med. 2017. PMID: 28002679 Clinical Trial.
-
Cost-effectiveness analysis of ocrelizumab versus subcutaneous interferon beta-1a for the treatment of relapsing multiple sclerosis.J Med Econ. 2017 Oct;20(10):1056-1065. doi: 10.1080/13696998.2017.1355310. Epub 2017 Jul 31. J Med Econ. 2017. PMID: 28703659
-
Ocrelizumab efficacy in subgroups of patients with relapsing multiple sclerosis.J Neurol. 2019 May;266(5):1182-1193. doi: 10.1007/s00415-019-09248-6. Epub 2019 Feb 28. J Neurol. 2019. PMID: 30820738 Free PMC article. Clinical Trial.
-
Efficacy, safety and patient reported outcomes in patients with active relapsing multiple sclerosis treated with ocrelizumab: Final results from the PRO-MSACTIVE study.Mult Scler Relat Disord. 2022 Dec;68:104109. doi: 10.1016/j.msard.2022.104109. Epub 2022 Aug 13. Mult Scler Relat Disord. 2022. PMID: 36007299 Review.
-
Ocrelizumab: A Review in Multiple Sclerosis.Drugs. 2022 Feb;82(3):323-334. doi: 10.1007/s40265-022-01672-9. Epub 2022 Feb 22. Drugs. 2022. PMID: 35192158 Free PMC article. Review.
Cited by
-
Impact of natalizumab on quality of life in a real-world cohort of patients with multiple sclerosis: Results from MS PATHS.Mult Scler J Exp Transl Clin. 2021 Apr 15;7(2):20552173211004634. doi: 10.1177/20552173211004634. eCollection 2021 Apr-Jun. Mult Scler J Exp Transl Clin. 2021. PMID: 33948221 Free PMC article.
-
[Ocrelizumab for treatment of multiple sclerosis].Nervenarzt. 2020 Aug;91(8):722-734. doi: 10.1007/s00115-020-00937-6. Nervenarzt. 2020. PMID: 32524163 Free PMC article. Review. German.
-
Clinical Trial Design-A Review-With Emphasis on Acute Intervertebral Disc Herniation.Front Vet Sci. 2020 Sep 2;7:583. doi: 10.3389/fvets.2020.00583. eCollection 2020. Front Vet Sci. 2020. PMID: 33134333 Free PMC article. Review.
-
Association of Higher Ocrelizumab Exposure With Reduced Disability Progression in Multiple Sclerosis.Neurol Neuroimmunol Neuroinflamm. 2023 Feb 15;10(2):e200094. doi: 10.1212/NXI.0000000000200094. Print 2023 Mar. Neurol Neuroimmunol Neuroinflamm. 2023. PMID: 36792367 Free PMC article. Clinical Trial.
-
Role of B Cells in Multiple Sclerosis and Related Disorders.Ann Neurol. 2021 Jan;89(1):13-23. doi: 10.1002/ana.25927. Epub 2020 Nov 4. Ann Neurol. 2021. PMID: 33091175 Free PMC article. Review.
References
-
- Calabresi PA, Kieseier BC, Arnold DL, et al. . Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol 2014;13:657–665. - PubMed
-
- Kappos L, Wiendl H, Selmaj K, et al. . Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2015;373:1418–1428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
