Subcortical heterotopic gray matter brain malformations: Classification study of 107 individuals
- PMID: 31484711
- PMCID: PMC6814414
- DOI: 10.1212/WNL.0000000000008200
Subcortical heterotopic gray matter brain malformations: Classification study of 107 individuals
Abstract
Objective: To better evaluate the imaging spectrum of subcortical heterotopic gray matter brain malformations (subcortical heterotopia [SUBH]), we systematically reviewed neuroimaging and clinical data of 107 affected individuals.
Methods: SUBH is defined as heterotopic gray matter, located within the white matter between the cortex and lateral ventricles. Four large brain malformation databases were searched for individuals with these malformations; data on imaging, clinical outcomes, and results of molecular testing were systematically reviewed and integrated with all previously published subtypes to create a single classification system.
Results: Review of the databases revealed 107 patients with SUBH, the large majority scanned during childhood (84%), including more than half before 4 years (59%). Although most individuals had cognitive or motor disability, 19% had normal development. Epilepsy was documented in 69%. Additional brain malformations were common and included abnormalities of the corpus callosum (65/102 [64%]), and, often, brainstem or cerebellum (47/106 [44%]). Extent of the heterotopic gray matter brain malformations (unilateral or bilateral) did not influence the presence or age at onset of seizures. Although genetic testing was not systematically performed in this group, the sporadic occurrence and frequent asymmetry suggests either postzygotic mutations or prenatal disruptive events. Several rare, bilateral forms are caused by mutations in genes associated with cell proliferation and polarity (EML1, TUBB, KATNB1, CENPJ, GPSM2).
Conclusion: This study reveals a broad clinical and imaging spectrum of heterotopic malformations and provides a framework for their classification.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
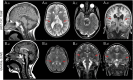
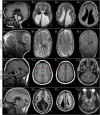
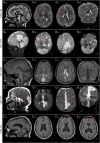
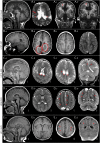
Similar articles
-
Gray matter heterotopia: clinical and neuroimaging report on 22 children.Acta Neurol Belg. 2022 Feb;122(1):153-162. doi: 10.1007/s13760-021-01774-3. Epub 2021 Sep 1. Acta Neurol Belg. 2022. PMID: 34471972 Free PMC article.
-
Bilateral posterior periventricular nodular heterotopia: a recognizable cortical malformation with a spectrum of associated brain abnormalities.AJNR Am J Neuroradiol. 2013 Feb;34(2):432-8. doi: 10.3174/ajnr.A3427. Epub 2013 Jan 24. AJNR Am J Neuroradiol. 2013. PMID: 23348762 Free PMC article.
-
Clinical and neuroimaging findings in children with gray matter heterotopias: A single institution experience of 36 patients.Eur J Paediatr Neurol. 2016 Sep;20(5):732-7. doi: 10.1016/j.ejpn.2016.05.009. Epub 2016 May 25. Eur J Paediatr Neurol. 2016. PMID: 27262615
-
Genetic malformations of the cerebral cortex and epilepsy.Epilepsia. 2005;46 Suppl 1:32-7. doi: 10.1111/j.0013-9580.2005.461010.x. Epilepsia. 2005. PMID: 15816977 Review.
-
Epilepsy and malformations of the cerebral cortex.Epileptic Disord. 2003 Sep;5 Suppl 2:S9-26. Epileptic Disord. 2003. PMID: 14617417 Review.
Cited by
-
International consensus recommendations on the diagnostic work-up for malformations of cortical development.Nat Rev Neurol. 2020 Nov;16(11):618-635. doi: 10.1038/s41582-020-0395-6. Epub 2020 Sep 7. Nat Rev Neurol. 2020. PMID: 32895508 Free PMC article. Review.
-
Congenital Visual Field Loss from a Schizencephalic Cleft Damaging Meyer's Loop.Neuroophthalmology. 2020 Nov 14;45(4):277-280. doi: 10.1080/01658107.2020.1844759. eCollection 2021. Neuroophthalmology. 2020. PMID: 34366518 Free PMC article.
-
Clinical and MRI findings of a suspected cortical malformation presented as a giant cerebral pseudomass in a German Shepherd dog.Clin Case Rep. 2023 Mar 8;11(3):e7057. doi: 10.1002/ccr3.7057. eCollection 2023 Mar. Clin Case Rep. 2023. PMID: 36911639 Free PMC article.
-
Definitions and classification of malformations of cortical development: practical guidelines.Brain. 2020 Oct 1;143(10):2874-2894. doi: 10.1093/brain/awaa174. Brain. 2020. PMID: 32779696 Free PMC article. Review.
-
Pediatric Brain Maturation and Migration Disorders.Diagnostics (Basel). 2022 Apr 30;12(5):1123. doi: 10.3390/diagnostics12051123. Diagnostics (Basel). 2022. PMID: 35626279 Free PMC article. Review.
References
-
- Harvey AS, Cross JH, Shinnar S, Mathern GW; ILAE Pediatric Epilepsy Surgery Survey Taskforce. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia 2008;49:146–155. - PubMed
-
- Hunter A. Brain. In: Stevenson RE, Hall JG, Goodman R, eds. Human Malformations and Related Anomalies. Oxford: Oxford University Press; 1993:1–52.
-
- Jacobs MP, Fischbach GD, Davis MR, et al. . Future directions for epilepsy research. Neurology 2001;57:1536–1542. - PubMed
-
- Kuzniecky R, Murro A, King D, et al. . Magnetic resonance imaging in childhood intractable partial epilepsies: pathologic correlations. Neurology 1993;43:681–687. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
