Sex differences by design and outcome in the Safety of Urate Elevation in PD (SURE-PD) trial
- PMID: 31484712
- PMCID: PMC6814412
- DOI: 10.1212/WNL.0000000000008194
Sex differences by design and outcome in the Safety of Urate Elevation in PD (SURE-PD) trial
Abstract
Objective: To investigate whether women and men with Parkinson disease (PD) differ in their biochemical and clinical responses to long-term treatment with inosine.
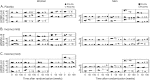
Methods: The Safety of Urate Elevation in Parkinson's Disease (SURE-PD) trial enrolled 75 people with early PD and baseline serum urate below 6 mg/dL and randomized them to 3 double-blinded treatment arms: oral placebo or inosine titrated to produce mild (6.1-7.0 mg/dL) or moderate (7.1-8.0 mg/dL) serum urate elevation for up to 2 years. Parkinsonism, serum urate, and plasma antioxidant capacity were measured at baseline and repeatedly on treatment; CSF urate was assessed once, at 3 months. Here in secondary analyses results are stratified by sex.
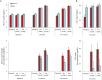
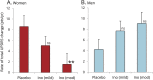
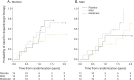
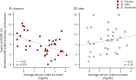
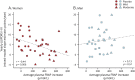
Results: Inosine produced an absolute increase in average serum urate from baseline that was 50% greater in women (3.0 mg/dL) than in men (2.0 mg/dL), consistent with expected lower baseline levels in women. Similarly, only among women was CSF urate significantly greater on mild or moderate inosine (+87% [p < 0.001] and +98% [p < 0.001], respectively) than on placebo (in contrast to men: +10% [p = 0.6] and +14% [p = 0.4], respectively). Women in the higher inosine dosing group showed a 7.0 Unified Parkinson's Disease Rating Scale (UPDRS) points/year lower rate of decline vs placebo (p = 0.01). In women, slower rates of UPDRS change were associated with greater increases in serum urate (r = -0.52; p = 0.001), and with greater increases in plasma antioxidant capacity (r = -0.44; p = 0.006). No significant associations were observed in men.
Conclusions: Inosine produced greater increases in serum and CSF urate in women compared to men in the SURE-PD trial, consistent with the study's design and with preliminary evidence for slower clinical decline in early PD among women treated with urate-elevating doses of inosine.
Clinicaltrialsgov identifier: NCT00833690.
Classification of evidence: This study provides Class II evidence that inosine produced greater urate elevation in women than men and may slow PD progression in women.
© 2019 American Academy of Neurology.
Figures
Comment in
-
Raising serum urate levels in Parkinson disease: A strategy only for women?Neurology. 2019 Oct 1;93(14):611-612. doi: 10.1212/WNL.0000000000008191. Epub 2019 Sep 4. Neurology. 2019. PMID: 31484716 No abstract available.
References
-
- Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid levels and the risk of idiopathic Parkinson's disease. Am J Epidemiol 1996;144:480–484. - PubMed
-
- de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol 2005;58:797–800. - PubMed
-
- Shen C, Guo Y, Luo W, Lin C, Ding M. Serum urate and the risk of Parkinson's disease: results from a meta-analysis. Can J Neurol Sci 2013;40:73–79. - PubMed
-
- Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol 2016;15:1257–1272. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
