Allosteric feedback inhibition of pyridoxine 5'-phosphate oxidase from Escherichia coli
- PMID: 31484724
- PMCID: PMC6816108
- DOI: 10.1074/jbc.RA119.009697
Allosteric feedback inhibition of pyridoxine 5'-phosphate oxidase from Escherichia coli
Abstract
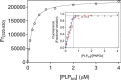
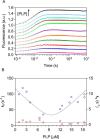
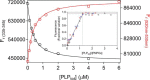
In Escherichia coli, the synthesis of pyridoxal 5'-phosphate (PLP), the catalytically active form of vitamin B6, takes place through the so-called deoxyxylulose 5-phosphate-dependent pathway, whose last step is pyridoxine 5'-phosphate (PNP) oxidation to PLP, catalyzed by the FMN-dependent enzyme PNP oxidase (PNPOx). This enzyme plays a pivotal role in controlling intracellular homeostasis and bioavailability of PLP. PNPOx has been proposed to undergo product inhibition resulting from PLP binding at the active site. PLP has also been reported to bind tightly at a secondary site, apparently without causing PNPOx inhibition. The possible location of this secondary site has been indicated by crystallographic studies as two symmetric surface pockets present on the PNPOx homodimer, but this site has never been verified by other experimental means. Here, we demonstrate, through kinetic measurements, that PLP inhibition is actually of a mixed-type nature and results from binding of this vitamer at an allosteric site. This interpretation was confirmed by the characterization of a mutated PNPOx form, in which substrate binding at the active site is heavily hampered but PLP binding is preserved. Structural and functional connections between the active site and the allosteric site were indicated by equilibrium binding experiments, which revealed different PLP-binding stoichiometries with WT and mutant PNPOx forms. These observations open up new horizons on the mechanisms that regulate E. coli PNPOx, which may have commonalities with the mechanisms regulating human PNPOx, whose crucial role in vitamin B6 metabolism and epilepsy is well-known.
Keywords: Escherichia coli; allosteric inhibition; allosteric regulation; enzyme mechanism; linear mixed-type inhibition; pyridoxal 5'-phosphate; pyridoxal phosphate; pyridoxine 5'-phosphate oxidase; vitamin; vitamin B6 biosynthesis.
© 2019 Barile et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




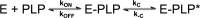
Similar articles
-
Pyridoxal 5'-Phosphate Biosynthesis by Pyridox-(am)-ine 5'-Phosphate Oxidase: Species-Specific Features.Int J Mol Sci. 2024 Mar 9;25(6):3174. doi: 10.3390/ijms25063174. Int J Mol Sci. 2024. PMID: 38542149 Free PMC article. Review.
-
Identification and characterization of the pyridoxal 5'-phosphate allosteric site in Escherichia coli pyridoxine 5'-phosphate oxidase.J Biol Chem. 2021 Jan-Jun;296:100795. doi: 10.1016/j.jbc.2021.100795. Epub 2021 May 18. J Biol Chem. 2021. PMID: 34019876 Free PMC article.
-
Structure and mechanism of Escherichia coli pyridoxine 5'-phosphate oxidase.Biochim Biophys Acta. 2003 Apr 11;1647(1-2):76-82. doi: 10.1016/s1570-9639(03)00060-8. Biochim Biophys Acta. 2003. PMID: 12686112 Review.
-
Tight binding of pyridoxal 5'-phosphate to recombinant Escherichia coli pyridoxine 5'-phosphate oxidase.Arch Biochem Biophys. 2000 May 1;377(1):109-14. doi: 10.1006/abbi.2000.1737. Arch Biochem Biophys. 2000. PMID: 10775448
-
X-ray structure of Escherichia coli pyridoxine 5'-phosphate oxidase complexed with FMN at 1.8 A resolution.Structure. 2000 Jul 15;8(7):751-62. doi: 10.1016/s0969-2126(00)00162-3. Structure. 2000. PMID: 10903950
Cited by
-
Protein engineering and iterative multimodule optimization for vitamin B6 production in Escherichia coli.Nat Commun. 2023 Aug 31;14(1):5304. doi: 10.1038/s41467-023-40928-0. Nat Commun. 2023. PMID: 37652926 Free PMC article.
-
Characterization of Novel Pathogenic Variants Causing Pyridox(am)ine 5'-Phosphate Oxidase-Dependent Epilepsy.Int J Mol Sci. 2021 Nov 6;22(21):12013. doi: 10.3390/ijms222112013. Int J Mol Sci. 2021. PMID: 34769443 Free PMC article.
-
Knowns and Unknowns of Vitamin B6 Metabolism in Escherichia coli.EcoSal Plus. 2021 Apr;9(2):eESP-0004-2021. doi: 10.1128/ecosalplus.ESP-0004-2021. EcoSal Plus. 2021. PMID: 33787481 Free PMC article. Review.
-
Pentylenetetrazole-Induced Seizures Are Increased after Kindling, Exhibiting Vitamin-Responsive Correlations to the Post-Seizures Behavior, Amino Acids Metabolism and Key Metabolic Regulators in the Rat Brain.Int J Mol Sci. 2023 Aug 3;24(15):12405. doi: 10.3390/ijms241512405. Int J Mol Sci. 2023. PMID: 37569781 Free PMC article.
-
Pyridoxal 5'-Phosphate Biosynthesis by Pyridox-(am)-ine 5'-Phosphate Oxidase: Species-Specific Features.Int J Mol Sci. 2024 Mar 9;25(6):3174. doi: 10.3390/ijms25063174. Int J Mol Sci. 2024. PMID: 38542149 Free PMC article. Review.
References
-
- di Salvo M. L., Safo M. K., and Contestabile R. (2012) Biomedical aspects of pyridoxal 5′-phosphate availability. Front. Biosci. (Elite Ed.) 4, 897–913 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

