Potential Role of Extracellular ATP Released by Bacteria in Bladder Infection and Contractility
- PMID: 31484739
- PMCID: PMC6731529
- DOI: 10.1128/mSphere.00439-19
Potential Role of Extracellular ATP Released by Bacteria in Bladder Infection and Contractility
Abstract
Urgency urinary incontinence (UUI) and overactive bladder (OAB) can both potentially be influenced by commensal and urinary tract infection-associated bacteria. The sensing of bladder filling involves interplay between various components of the nervous system, eventually resulting in contraction of the detrusor muscle during micturition. This study models host responses to various urogenital bacteria, first by using urothelial bladder cell lines and then with myofibroblast contraction assays. To measure responses, we examined Ca2+ influx, gene expression, and alpha smooth muscle actin deposition assays. Organisms such as Escherichia coli and Gardnerella vaginalis were found to strongly induce Ca2+ influx and contraction, whereas Lactobacillus crispatus and L. gasseri did not induce this response. Additionally, supernatants from lactobacilli impeded Ca2+ influx and contraction induced by uropathogens. Upon further investigation of factors associated with purinergic signaling pathways, the Ca2+ influx and contraction of cells correlated with the amount of extracellular ATP produced by E. coli Certain lactobacilli appear to mitigate this response by utilizing extracellular ATP or producing inhibitory compounds that may act as a receptor agonist or Ca2+ channel blocker. These findings suggest that members of the urinary microbiota may be influencing UUI or OAB.IMPORTANCE The ability of uropathogenic bacteria to release excitatory compounds, such as ATP, may act as a virulence factor to stimulate signaling pathways that could have profound effects on the urothelium, perhaps extending to the vagina. This may be countered by the ability of certain commensal urinary microbiota constituents, such as lactobacilli. Further understanding of these interactions is important for the treatment and prevention of UUI and OAB. The clinical implications may require a more targeted approach to enhance the commensal bacteria and reduce ATP release by pathogens.
Keywords: ATP; Escherichia coli; Gardnerella; Lactobacillus; extracellular.
Copyright © 2019 Abbasian et al.
Figures
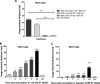







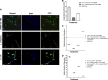
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
