Home initiation of chronic non-invasive ventilation in COPD patients with chronic hypercapnic respiratory failure: a randomised controlled trial
- PMID: 31484786
- PMCID: PMC7063397
- DOI: 10.1136/thoraxjnl-2019-213303
Home initiation of chronic non-invasive ventilation in COPD patients with chronic hypercapnic respiratory failure: a randomised controlled trial
Abstract
Introduction: Chronic non-invasive ventilation (NIV) has become evidence-based care for stable hypercapnic COPD patients. While the number of patients increases, home initiation of NIV would greatly alleviate the healthcare burden. We hypothesise that home initiation of NIV with the use of telemedicine in stable hypercapnic COPD is non-inferior to in-hospital NIV initiation.
Methods: Sixty-seven stable hypercapnic COPD patients were randomised to initiation of NIV in the hospital or at home using telemedicine. Primary outcome was daytime arterial carbon dioxide pressure (PaCO2) reduction after 6 months NIV, with a non-inferiority margin of 0.4 kPa. Secondary outcomes were health-related quality of life (HRQoL) and costs.
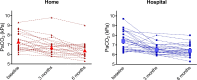
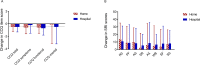
Results: Home NIV initiation was non-inferior to in-hospital initiation (adjusted mean difference in PaCO2 change home vs in-hospital: 0.04 kPa (95% CI -0.31 to 0.38 kPa), with both groups showing a PaCO2 reduction at 6 months compared with baseline (home: from 7.3±0.9 to 6.4±0.8 kPa (p<0.001) and in-hospital: from 7.4±1.0 to 6.4±0.6 kPa (p<0.001)). In both groups, HRQoL improved without a difference in change between groups (Clinical COPD Questionnaire total score-adjusted mean difference 0.0 (95% CI -0.4 to 0.5)). Furthermore, home NIV initiation was significantly cheaper (home: median €3768 (IQR €3546-€4163) vs in-hospital: median €8537 (IQR €7540-€9175); p<0.001).
Discussion: This is the first study showing that home initiation of chronic NIV in stable hypercapnic COPD patients, with the use of telemedicine, is non-inferior to in-hospital initiation, safe and reduces costs by over 50%.
Trial registration number: NCT02652559.
Keywords: COPD; non-invasive ventilation; telemedicine.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: MLD reports a grant from the Dutch Lung Foundation (junior investigator grant number 5.2.15.057JO) and the Philips Respironics, Murrysville, Pennsylvania, USA, provided for the conduct of the present study. Furthermore, MLD received grants and personal fees from Vivisol B.V., grants from Fisher and Paykel Ltd, and personal fees from Philips, outside the submitted work. JMV, GB, AH, JN, JPvM and JFMvB have no competing interests. HAMK reports an unrestricted research grant and fees for participation in advisory boards from Boehringer Ingelheim, Novartis and GlaxoSmithKline, and fees for advisory board participation from AstraZeneca and Chiesi, all above paid to his institution and outside the submitted work. PJW reports grants and personal fees from Philips and RESMED, grants from Vital Air, VIVISOL and Goedegebuure, and personal fees from Synapse and Bresotec, all outside the submitted work.
Figures


Comment in
-
No place like home: initiation of non-invasive ventilation for stable severe COPD.Thorax. 2020 Mar;75(3):196-197. doi: 10.1136/thoraxjnl-2019-213787. Epub 2020 Jan 29. Thorax. 2020. PMID: 31996402 No abstract available.
-
Critically appraised paper: In people with chronic obstructive pulmonary disease, initiation of nocturnal non-invasive ventilation at home is non-inferior to initiation during a hospital admission [commentary].J Physiother. 2020 Oct;66(4):267. doi: 10.1016/j.jphys.2020.07.007. Epub 2020 Aug 23. J Physiother. 2020. PMID: 32847763 Free PMC article. No abstract available.
-
Critically appraised paper: In people with chronic obstructive pulmonary disease, initiation of nocturnal non-invasive ventilation at home is non-inferior to initiation during a hospital admission [synopsis].J Physiother. 2020 Oct;66(4):267. doi: 10.1016/j.jphys.2020.07.009. Epub 2020 Aug 25. J Physiother. 2020. PMID: 32859567 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
