Clinical value of a plasma Epstein-Barr virus DNA assay in the diagnosis of recurrent or metastatic nasopharyngeal carcinoma: a meta-analysis
- PMID: 31484795
- PMCID: PMC6753325
- DOI: 10.1042/BSR20190691
Clinical value of a plasma Epstein-Barr virus DNA assay in the diagnosis of recurrent or metastatic nasopharyngeal carcinoma: a meta-analysis
Abstract
Background: To evaluate the diagnostic value of Epstein-Barr virus (EBV) DNA in nasopharyngeal carcinoma (NPC) patients with locoregional or distant recurrence.
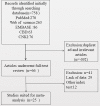
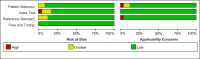
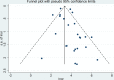
Methods: Articles related to the diagnosis of recurrent or metastatic NPC by the detection of EBV DNA in plasma or serum were retrieved from different databases. Sensitivity, specificity, summary receiver operating characteristic (SROC) curves, and likelihood ratios were pooled to assess the diagnostic value of individual diagnostic tests.
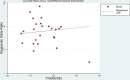
Results: This meta-analysis pooled 25 eligible studies including 2496 patients with NPC. The sensitivity, specificity, positive likelihood ratio (+LR), and negative likelihood ratio (-LR) of EBV DNA in the diagnosis of NPC were 0.858 (95% confidence interval (CI): 0.801-0.901), 0.890 (95% CI: 0.866-0.909), 7.782 (95% CI: 6.423-9.429) and 0.159 (95% CI: 0.112-0.226), respectively. The diagnostic odds ratio (DOR) was 48.865 (95% CI: 31.903-74.845). The SROC for EBV DNA detection was 0.93 (95% CI: 0.90-0.95).
Conclusion: The detection of EBV DNA for the diagnosis of recurrent or metastatic NPC has good sensitivity and specificity and might be helpful in monitoring recurrent or metastatic NPC.
Keywords: Epstein-Barr Virus DNA; Meta-Analysis; metastatic; nasopharyngeal carcinoma; recurrent.
© 2019 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures




Similar articles
-
Prognostic values of the integrated model incorporating the volume of metastatic regional cervical lymph node and pretreatment serum Epstein-Barr virus DNA copy number in predicting distant metastasis in patients with N1 nasopharyngeal carcinoma.Chin J Cancer. 2017 Dec 29;36(1):98. doi: 10.1186/s40880-017-0264-x. Chin J Cancer. 2017. PMID: 29284539 Free PMC article.
-
Liquid biopsy with plasma Epstein-Barr virus DNA characterizes biological relapse for the prediction of cancer recurrence in non-disseminated nasopharyngeal carcinoma.Eur J Cancer. 2024 Dec;213:115098. doi: 10.1016/j.ejca.2024.115098. Epub 2024 Oct 26. Eur J Cancer. 2024. PMID: 39486162
-
Prognostic value of Epstein-Barr virus DNA level for nasopharyngeal carcinoma: a meta-analysis of 8128 cases.Eur Arch Otorhinolaryngol. 2020 Jan;277(1):9-18. doi: 10.1007/s00405-019-05699-9. Epub 2019 Oct 28. Eur Arch Otorhinolaryngol. 2020. PMID: 31659449 Review.
-
Prognostic value of gross tumor regression and plasma Epstein Barr Virus DNA levels at the end of intensity-modulated radiation therapy in patients with nasopharyngeal carcinoma.Radiother Oncol. 2019 Mar;132:223-229. doi: 10.1016/j.radonc.2018.10.010. Epub 2018 Oct 23. Radiother Oncol. 2019. PMID: 30366725
-
Systematic review on Epstein-Barr virus (EBV) DNA in diagnosis of nasopharyngeal carcinoma in Asian populations.Asian Pac J Cancer Prev. 2012;13(6):2577-81. doi: 10.7314/apjcp.2012.13.6.2577. Asian Pac J Cancer Prev. 2012. PMID: 22938423
Cited by
-
The Promise of Circulating Tumor DNA in Head and Neck Cancer.Cancers (Basel). 2022 Jun 16;14(12):2968. doi: 10.3390/cancers14122968. Cancers (Basel). 2022. PMID: 35740633 Free PMC article. Review.
-
Chronic Hepatitis Virus Infection Are Associated With High Risk of Gastric Cancer: A Systematic Review and Cumulative Analysis.Front Oncol. 2021 Jul 8;11:703558. doi: 10.3389/fonc.2021.703558. eCollection 2021. Front Oncol. 2021. PMID: 34307172 Free PMC article.
-
Extraction parameter optimized radiomics for neoadjuvant chemotherapy response prognosis in advanced nasopharyngeal carcinoma.Clin Transl Radiat Oncol. 2021 Dec 29;33:37-44. doi: 10.1016/j.ctro.2021.12.005. eCollection 2022 Mar. Clin Transl Radiat Oncol. 2021. PMID: 35024463 Free PMC article.
-
Japan society of clinical oncology position paper on appropriate clinical use of molecular residual disease (MRD) testing.Int J Clin Oncol. 2025 Apr;30(4):605-654. doi: 10.1007/s10147-024-02683-0. Epub 2025 Feb 7. Int J Clin Oncol. 2025. PMID: 39920551 Free PMC article. Review.
-
SEOM-TTCC clinical guideline in nasopharynx cancer (2021).Clin Transl Oncol. 2022 Apr;24(4):670-680. doi: 10.1007/s12094-022-02814-x. Epub 2022 Mar 18. Clin Transl Oncol. 2022. PMID: 35303267 Free PMC article.
References
-
- Marks J.E., Phillips J.L. and Menck H.R. (1998) The National Cancer Data Base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer 83, 582–588 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

