Predicting the broadly neutralizing antibody susceptibility of the HIV reservoir
- PMID: 31484826
- PMCID: PMC6777915
- DOI: 10.1172/jci.insight.130153
Predicting the broadly neutralizing antibody susceptibility of the HIV reservoir
Abstract
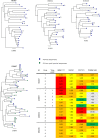
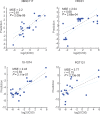
Broadly neutralizing antibodies (bNAbs) against HIV-1 are under evaluation for both prevention and therapy. HIV-1 sequence diversity observed in most HIV-infected individuals and archived variations in critical bNAb epitopes present a major challenge for the clinical application of bNAbs, as preexistent resistant viral strains can emerge, resulting in bNAb failure to control HIV. In order to identify viral resistance in patients prior to antibody therapy and to guide the selection of effective bNAb combination regimens, we developed what we believe to be a novel Bayesian machine-learning model that uses HIV-1 envelope protein sequences and foremost approximated glycan occupancy information as variables to quantitatively predict the half-maximal inhibitory concentrations (IC50) of 126 neutralizing antibodies against a variety of cross clade viruses. We then applied this model to peripheral blood mononuclear cell-derived proviral Env sequences from 25 HIV-1-infected individuals mapping the landscape of neutralization resistance within each individual's reservoir and determined the predicted ideal bNAb combination to achieve 100% neutralization at IC50 values <1 μg/ml. Furthermore, predicted cellular viral reservoir neutralization signatures of individuals before an analytical antiretroviral treatment interruption were consistent with the measured neutralization susceptibilities of the respective plasma rebound viruses, validating our model as a potentially novel tool to facilitate the advancement of bNAbs into the clinic.
Keywords: AIDS/HIV; Bioinformatics; Diagnostics; Immunotherapy; Therapeutics.
Conflict of interest statement
Figures





Similar articles
-
Neutralizing Activity of Broadly Neutralizing Anti-HIV-1 Antibodies against Clade B Clinical Isolates Produced in Peripheral Blood Mononuclear Cells.J Virol. 2018 Feb 12;92(5):e01883-17. doi: 10.1128/JVI.01883-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237833 Free PMC article.
-
Landscape of Human Immunodeficiency Virus Neutralization Susceptibilities Across Tissue Reservoirs.Clin Infect Dis. 2022 Oct 12;75(8):1342-1350. doi: 10.1093/cid/ciac164. Clin Infect Dis. 2022. PMID: 35234862 Free PMC article.
-
Characterizing the Relationship Between Neutralization Sensitivity and env Gene Diversity During ART Suppression.Front Immunol. 2021 Sep 15;12:710327. doi: 10.3389/fimmu.2021.710327. eCollection 2021. Front Immunol. 2021. PMID: 34603284 Free PMC article.
-
Antiviral Therapy by HIV-1 Broadly Neutralizing and Inhibitory Antibodies.Int J Mol Sci. 2016 Nov 18;17(11):1901. doi: 10.3390/ijms17111901. Int J Mol Sci. 2016. PMID: 27869733 Free PMC article. Review.
-
Current methods for detecting and assessing HIV-1 antibody resistance.Front Immunol. 2025 Jan 6;15:1443377. doi: 10.3389/fimmu.2024.1443377. eCollection 2024. Front Immunol. 2025. PMID: 39835119 Free PMC article. Review.
Cited by
-
Potential neutralizing antibodies discovered for novel corona virus using machine learning.Sci Rep. 2021 Mar 4;11(1):5261. doi: 10.1038/s41598-021-84637-4. Sci Rep. 2021. PMID: 33664393 Free PMC article.
-
Hitting the sweet spot: exploiting HIV-1 glycan shield for induction of broadly neutralizing antibodies.Curr Opin HIV AIDS. 2020 Sep;15(5):267-274. doi: 10.1097/COH.0000000000000639. Curr Opin HIV AIDS. 2020. PMID: 32675574 Free PMC article. Review.
-
Subtle Longitudinal Alterations in Env Sequence Potentiate Differences in Sensitivity to Broadly Neutralizing Antibodies following Acute HIV-1 Subtype C Infection.J Virol. 2022 Dec 21;96(24):e0127022. doi: 10.1128/jvi.01270-22. Epub 2022 Dec 1. J Virol. 2022. PMID: 36453881 Free PMC article.
-
Development of screening assays for use of broadly neutralizing antibodies in people with HIV.Curr Opin HIV AIDS. 2023 Jul 1;18(4):171-177. doi: 10.1097/COH.0000000000000798. Epub 2023 May 9. Curr Opin HIV AIDS. 2023. PMID: 37265260 Free PMC article. Review.
-
Prevalence of resistance-associated viral variants to the HIV-specific broadly neutralising antibody 10-1074 in a UK bNAb-naïve population.Front Immunol. 2024 Mar 18;15:1352123. doi: 10.3389/fimmu.2024.1352123. eCollection 2024. Front Immunol. 2024. PMID: 38562938 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK108056/DK/NIDDK NIH HHS/United States
- U01 CA215798/CA/NCI NIH HHS/United States
- R21 AI140788/AI/NIAID NIH HHS/United States
- UM1 AI126603/AI/NIAID NIH HHS/United States
- R37 AI080289/AI/NIAID NIH HHS/United States
- U19 AI142790/AI/NIAID NIH HHS/United States
- R01 AI138790/AI/NIAID NIH HHS/United States
- K08 AI106408/AI/NIAID NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- U19 AI128751/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- R01 AI080289/AI/NIAID NIH HHS/United States
- R01 AI129797/AI/NIAID NIH HHS/United States
- U54 CA210180/CA/NCI NIH HHS/United States
- U54 CA217377/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

