Suboptimal hydration remodels metabolism, promotes degenerative diseases, and shortens life
- PMID: 31484829
- PMCID: PMC6777918
- DOI: 10.1172/jci.insight.130949
Suboptimal hydration remodels metabolism, promotes degenerative diseases, and shortens life
Abstract
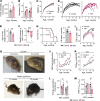
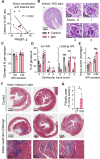
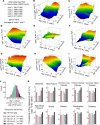
With increased life expectancy worldwide, there is an urgent need for improving preventive measures that delay the development of age-related degenerative diseases. Here, we report evidence from mouse and human studies that this goal can be achieved by maintaining optimal hydration throughout life. We demonstrate that restricting the amount of drinking water shortens mouse lifespan with no major warning signs up to 14 months of life, followed by sharp deterioration. Mechanistically, water restriction yields stable metabolism remodeling toward metabolic water production with greater food intake and energy expenditure, an elevation of markers of inflammation and coagulation, accelerated decline of neuromuscular coordination, renal glomerular injury, and the development of cardiac fibrosis. In humans, analysis of data from the Atherosclerosis Risk in Communities (ARIC) study revealed that hydration level, assessed at middle age by serum sodium concentration, is associated with markers of coagulation and inflammation and predicts the development of many age-related degenerative diseases 24 years later. The analysis estimates that improving hydration throughout life may greatly decrease the prevalence of degenerative diseases, with the most profound effect on dementia, heart failure (HF), and chronic lung disease (CLD), translating to the development of these diseases in 3 million fewer people in the United States alone.
Keywords: Aging; Epidemiology.
Conflict of interest statement
Figures


References
-
- Jéquier E, Constant F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr. 2010;64(2):115–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

