Protein prenylation restrains innate immunity by inhibiting Rac1 effector interactions
- PMID: 31484924
- PMCID: PMC6726657
- DOI: 10.1038/s41467-019-11606-x
Protein prenylation restrains innate immunity by inhibiting Rac1 effector interactions
Abstract
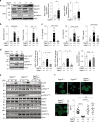
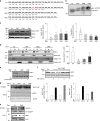
Rho family proteins are prenylated by geranylgeranyltransferase type I (GGTase-I), which normally target proteins to membranes for GTP-loading. However, conditional deletion of GGTase-I in mouse macrophages increases GTP-loading of Rho proteins, leading to enhanced inflammatory responses and severe rheumatoid arthritis. Here we show that heterozygous deletion of the Rho family gene Rac1, but not Rhoa and Cdc42, reverses inflammation and arthritis in GGTase-I-deficient mice. Non-prenylated Rac1 has a high affinity for the adaptor protein Ras GTPase-activating-like protein 1 (Iqgap1), which facilitates both GTP exchange and ubiquitination-mediated degradation of Rac1. Consistently, inactivating Iqgap1 normalizes Rac1 GTP-loading, and reduces inflammation and arthritis in GGTase-I-deficient mice, as well as prevents statins from increasing Rac1 GTP-loading and cytokine production in macrophages. We conclude that blocking prenylation stimulates Rac1 effector interactions and unleashes proinflammatory signaling. Our results thus suggest that prenylation normally restrains innate immune responses by preventing Rac1 effector interactions.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Geranylgeranyltransferase type I (GGTase-I) deficiency hyperactivates macrophages and induces erosive arthritis in mice.J Clin Invest. 2011 Feb;121(2):628-39. doi: 10.1172/JCI43758. Epub 2011 Jan 25. J Clin Invest. 2011. PMID: 21266780 Free PMC article.
-
Geranylgeranyltransferase I promotes human glioma cell growth through Rac1 membrane association and activation.J Mol Neurosci. 2013 Jan;49(1):130-9. doi: 10.1007/s12031-012-9905-3. Epub 2012 Oct 17. J Mol Neurosci. 2013. PMID: 23073905
-
Inhibiting PGGT1B Disrupts Function of RHOA, Resulting in T-cell Expression of Integrin α4β7 and Development of Colitis in Mice.Gastroenterology. 2019 Nov;157(5):1293-1309. doi: 10.1053/j.gastro.2019.07.007. Epub 2019 Jul 11. Gastroenterology. 2019. PMID: 31302143
-
New model for the interaction of IQGAP1 with CDC42 and RAC1.Small GTPases. 2020 Jan;11(1):16-22. doi: 10.1080/21541248.2017.1321169. Epub 2017 Jun 19. Small GTPases. 2020. PMID: 28622072 Free PMC article. Review.
-
Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases.Curr Opin Cell Biol. 1999 Oct;11(5):591-6. doi: 10.1016/s0955-0674(99)00014-9. Curr Opin Cell Biol. 1999. PMID: 10508646 Review.
Cited by
-
The E3 ubiquitin ligase MG53 inhibits hepatocellular carcinoma by targeting RAC1 signaling.Oncogenesis. 2022 Jul 20;11(1):40. doi: 10.1038/s41389-022-00414-6. Oncogenesis. 2022. PMID: 35858925 Free PMC article.
-
Targeting protein modifications in metabolic diseases: molecular mechanisms and targeted therapies.Signal Transduct Target Ther. 2023 May 27;8(1):220. doi: 10.1038/s41392-023-01439-y. Signal Transduct Target Ther. 2023. PMID: 37244925 Free PMC article. Review.
-
E3 Ubiquitin Ligase TRIP12 Controls Exit from Mitosis via Positive Regulation of MCL-1 in Response to Taxol.Cancers (Basel). 2023 Jan 13;15(2):505. doi: 10.3390/cancers15020505. Cancers (Basel). 2023. PMID: 36672454 Free PMC article.
-
Geranylgeraniol Restores Zoledronic Acid-Induced Efferocytosis Inhibition in Bisphosphonate-Related Osteonecrosis of the Jaw.Front Cell Dev Biol. 2021 Nov 3;9:770899. doi: 10.3389/fcell.2021.770899. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34805177 Free PMC article.
-
Actin Remodeling Defects Leading to Autoinflammation and Immune Dysregulation.Front Immunol. 2021 Jan 7;11:604206. doi: 10.3389/fimmu.2020.604206. eCollection 2020. Front Immunol. 2021. PMID: 33488606 Free PMC article. Review.
References
-
- Hori Y, et al. Post-translational modifications of the C-terminal region of the rho protein are important for its interaction with membranes and the stimulatory and inhibitory GDP/GTP exchange proteins. Oncogene. 1991;6:515–522. - PubMed
-
- Sahai E, Marshall CJ. Rho-GTPases and cancer. Nat. Rev. Cancer. 2002;2:133–142. - PubMed
-
- Kusama T, et al. Selective inhibition of cancer cell invasion by a geranylgeranyltransferase-I inhibitor. Clin. Exp. Metastasis. 2003;20:561–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

