Phospholipase C-related catalytically inactive protein regulates cytokinesis by protecting phosphatidylinositol 4,5-bisphosphate from metabolism in the cleavage furrow
- PMID: 31484968
- PMCID: PMC6726632
- DOI: 10.1038/s41598-019-49156-3
Phospholipase C-related catalytically inactive protein regulates cytokinesis by protecting phosphatidylinositol 4,5-bisphosphate from metabolism in the cleavage furrow
Abstract
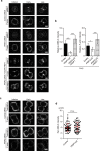
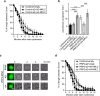
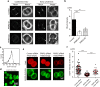
Cytokinesis is initiated by the formation and ingression of the cleavage furrow. Phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] accumulation followed by RhoA translocation to the cleavage furrow are prerequisites for cytokinesis progression. Here, we investigated whether phospholipase C (PLC)-related catalytically inactive protein (PRIP), a metabolic modulator of PI(4,5)P2, regulates PI(4,5)P2-mediated cytokinesis. We found that PRIP localised to the cleavage furrow during cytokinesis. Moreover, HeLa cells with silenced PRIP displayed abnormal cytokinesis. Importantly, PI(4,5)P2 accumulation at the cleavage furrow, as well as the localisation of RhoA and phospho-myosin II regulatory light chain to the cleavage furrow, were reduced in PRIP-silenced cells. The overexpression of oculocerebrorenal syndrome of Lowe-1 (OCRL1), a phosphatidylinositol-5-phosphatase, in cells decreased PI(4,5)P2 levels during early cytokinesis and resulted in cytokinesis abnormalities. However, these abnormal cytokinesis phenotypes were ameliorated by the co-expression of PRIP but not by co-expression of a PI(4,5)P2-unbound PRIP mutant. Collectively, our results indicate that PRIP is a component at the cleavage furrow that maintains PI(4,5)P2 metabolism and regulates RhoA-dependent progression of cytokinesis. Thus, we propose that PRIP regulates phosphoinositide metabolism correctively and mediates normal cytokinesis progression.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

