Salmonella persisters promote the spread of antibiotic resistance plasmids in the gut
- PMID: 31485077
- PMCID: PMC6744281
- DOI: 10.1038/s41586-019-1521-8
Salmonella persisters promote the spread of antibiotic resistance plasmids in the gut
Abstract
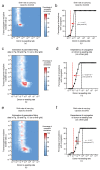
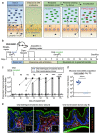
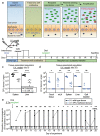
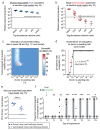
The emergence of antibiotic-resistant bacteria through mutations or the acquisition of genetic material such as resistance plasmids represents a major public health issue1,2. Persisters are subpopulations of bacteria that survive antibiotics by reversibly adapting their physiology3-10, and can promote the emergence of antibiotic-resistant mutants11. We investigated whether persisters can also promote the spread of resistance plasmids. In contrast to mutations, the transfer of resistance plasmids requires the co-occurrence of both a donor and a recipient bacterial strain. For our experiments, we chose the facultative intracellular entero-pathogen Salmonella enterica serovar Typhimurium (S. Typhimurium) and Escherichia coli, a common member of the microbiota12. S. Typhimurium forms persisters that survive antibiotic therapy in several host tissues. Here we show that tissue-associated S. Typhimurium persisters represent long-lived reservoirs of plasmid donors or recipients. The formation of reservoirs of S. Typhimurium persisters requires Salmonella pathogenicity island (SPI)-1 and/or SPI-2 in gut-associated tissues, or SPI-2 at systemic sites. The re-seeding of these persister bacteria into the gut lumen enables the co-occurrence of donors with gut-resident recipients, and thereby favours plasmid transfer between various strains of Enterobacteriaceae. We observe up to 99% transconjugants within two to three days of re-seeding. Mathematical modelling shows that rare re-seeding events may suffice for a high frequency of conjugation. Vaccination reduces the formation of reservoirs of persisters after oral infection with S. Typhimurium, as well as subsequent plasmid transfer. We conclude that-even without selection for plasmid-encoded resistance genes-small reservoirs of pathogen persisters can foster the spread of promiscuous resistance plasmids in the gut.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Comment in
-
Spreading resistance in Salmonella's sleep.Nat Rev Microbiol. 2019 Nov;17(11):645. doi: 10.1038/s41579-019-0272-8. Nat Rev Microbiol. 2019. PMID: 31537913 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

