Establishment of maternal blood supply to the placenta: insights into plugging, unplugging and trophoblast behaviour from an agent-based model
- PMID: 31485310
- PMCID: PMC6710655
- DOI: 10.1098/rsfs.2019.0019
Establishment of maternal blood supply to the placenta: insights into plugging, unplugging and trophoblast behaviour from an agent-based model
Abstract
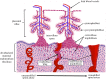
The ability of the baby to receive nutrients and oxygen in utero depends on the healthy development of the placenta. For maternal blood to adequately perfuse the placenta, it dramatically alters the arteries in the uterus that supply it with nutrient-rich blood right from the start of pregnancy. Placental cells (trophoblasts) invade both into the tissue of the uterus and into the maternal blood vessels nearest to the site of implantation (the spiral arteries (SAs)) and transform these allowing a relatively high and steady flow of nutrient-rich blood to perfuse the placenta. Trophoblasts also form plugs that occlude SAs, preventing maternal blood flow to the placenta until the late first trimester, at which point these plugs dislodge or disintegrate. Here we present an agent-based model of trophoblast migration within plugged SAs to tease apart the impact of chemical signals and mechanical factors on trophoblast behaviour. The model supports our previous in vitro hypothesis that plugging of the maternal arteries in early pregnancy can act to promote trophoblast invasion by providing a 'low flow' environment and extends our understanding by suggesting 'weak spots' in plug structure can lead to plug degeneration, allowing increased blood flow through the materno-fetal circulation.
Keywords: agent-based model; haemodynamics; placenta; spiral artery; trophoblast; utero-placental circulation.
Conflict of interest statement
We declare we have no competing interests.
Figures






References
-
- Benirschke K, Kaufmann P, Baergen R. 2000. Pathology of the human placenta. New York, NY: Springer.
-
- Cunninham F. 2005. Implantation, embryogenesis, and placental development. In Williams obstetrics, 22nd edn, pp. 39–90. New York, NY: McGraw-Hill.
Associated data
LinkOut - more resources
Full Text Sources
