Mechanics of cervical remodelling: insights from rodent models of pregnancy
- PMID: 31485313
- PMCID: PMC6710664
- DOI: 10.1098/rsfs.2019.0026
Mechanics of cervical remodelling: insights from rodent models of pregnancy
Abstract
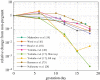
The uterine cervix undergoes a complex remodelling process during pregnancy, characterized by dramatic changes in both extracellular matrix (ECM) structure and mechanical properties. Understanding the cervical remodelling process in a term or preterm birth will aid efforts for the prevention of preterm births (PTBs), which currently affect 14.8 million babies annually worldwide. Animal models of pregnancy, particularly rodents, continue to provide valuable insights into the cervical remodelling process, through the study of changes in ECM structure and mechanical properties at defined gestation time points. Currently, there is a lack of a collective, quantitative framework to relate the complex, nonlinear mechanical behaviour of the rodent cervix to changes in ECM structure. This review aims to fill this gap in knowledge by outlining the current understanding of cervical remodelling during pregnancy in rodent models in the context of solid biomechanics. Here we highlight the collective contribution of multiple mechanical studies which give evidence that cervical softening coincides with known ECM changes throughout pregnancy. Taken together, mechanical tests on tissue from pregnant rodents reveal the cervix's remarkable ability to soften dramatically during gestation to allow for a compliant tissue that can withstand damage and can dissipate mechanical loads.
Keywords: cervix; extracellular matrix (ECM); growth and remodelling; review; soft tissue mechanics.
Conflict of interest statement
The authors have no competing interests to disclose.
Figures
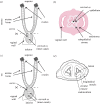

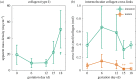
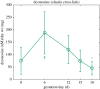
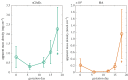



References
-
- Centers for Disease Control and Prevention, Preterm birth, 2018. See https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

