Effects of curcumin on oxidative stress, inflammation and apoptosis in L-arginine induced acute pancreatitis in mice
- PMID: 31485503
- PMCID: PMC6717142
- DOI: 10.1016/j.heliyon.2019.e02222
Effects of curcumin on oxidative stress, inflammation and apoptosis in L-arginine induced acute pancreatitis in mice
Abstract
Background and purpose: Curcumin, an active constituent of rhizomes of Curcuma longa Linn, exhibits a variety of biological activities such as anti-inflammation and anti-oxidant. The present study aims to examine the effects of curcumin on oxidative stress, inflammation and apoptosis in L-arginine induced acute pancreatitis (AP) in mice.
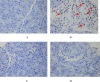
Methods: Male ICR mice were randomly divided into 4 groups. Control group received intraperitoneal injection (i.p.) of 1% DMSO as a vehicle. AP group received two doses of i.p. L-arginine (L-Arg) 450 mg/100 g body weight (BW) at 1-hour interval. AP plus low-dose curcumin group received i.p. curcumin 50 mg/kg BW 1 hour before L-Arg injection and then once daily for 3 days. AP plus high-dose curcumin group received i.p. curcumin 200 mg/kg BW 1 hour before L-Arg injection and then once daily for 3 days. All mice were sacrificed at 72 hours. Pancreatic tissue was obtained for histological evaluation, immunohistochemical studies for nuclear factor-kappa beta (NF-kβ), apoptosis and myeloperoxidase (MPO), and Western blot analyses for 4-Hydroxynonenal (4-HNE). Blood samples were collected for amylase analysis.
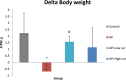
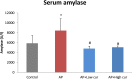
Results: Mean body weight was significantly lower in AP group than in control group, while in curcumin group, body weight was maintained. The serum amylase, number of MPO positive cells, NF-kB positive cells, TUNEL positive cells, and 4-HNE expression significantly increased in AP group when compared with control group, but decreased in low and high-dose curcumin groups. Mice in AP group developed severe pancreatic inflammation, edema and fat necrosis. While mice in low and high-dose curcumin groups showed a significant improvement in histopathological scores. There was no significant difference between low and high doses of curcumin.
Conclusion: Curcumin could attenuate acute pancreatitis via anti-oxidant, anti-inflammation and anti-apoptosis property leading to the improvement in pancreatic damage.
Keywords: 4-HNE; Apoptosis; Biochemistry; Cell biology; Curcumin; Myeloperoxidase; Pancreatitis; Physiology; Systems biology.
Figures







Similar articles
-
Genistein attenuated oxidative stress, inflammation, and apoptosis in L-arginine induced acute pancreatitis in mice.BMC Complement Med Ther. 2022 Aug 4;22(1):208. doi: 10.1186/s12906-022-03689-9. BMC Complement Med Ther. 2022. PMID: 35927726 Free PMC article.
-
Effects of probiotics on pancreatic inflammation and intestinal integrity in mice with acute pancreatitis.BMC Complement Med Ther. 2023 May 22;23(1):166. doi: 10.1186/s12906-023-03998-7. BMC Complement Med Ther. 2023. PMID: 37217916 Free PMC article.
-
Ameliorative Effects of Curcumin on Fibrinogen-Like Protein-2 Gene Expression, Some Oxido-Inflammatory and Apoptotic Markers in a Rat Model of l-Arginine-Induced Acute Pancreatitis.J Biochem Mol Toxicol. 2016 Jun;30(6):302-8. doi: 10.1002/jbt.21794. Epub 2016 Feb 10. J Biochem Mol Toxicol. 2016. PMID: 26862043
-
Anti-inflammatory activity of curcumin in a model of L-arginine-induced acute pancreatitis in rats.Ann Ital Chir. 2023 May 8;12:S2239253X2303880X. Ann Ital Chir. 2023. PMID: 37199116
-
Anti-inflammatory and Antioxidant Effects of Captopril Compared to Methylprednisolone in L-Arginine-Induced Acute Pancreatitis.Dig Dis Sci. 2018 Jun;63(6):1497-1505. doi: 10.1007/s10620-018-5036-1. Epub 2018 Mar 29. Dig Dis Sci. 2018. PMID: 29594979
Cited by
-
Curcumin Regulates Cancer Progression: Focus on ncRNAs and Molecular Signaling Pathways.Front Oncol. 2021 Apr 12;11:660712. doi: 10.3389/fonc.2021.660712. eCollection 2021. Front Oncol. 2021. PMID: 33912467 Free PMC article. Review.
-
Curcumin for gastric cancer: Mechanism prediction via network pharmacology, docking, and in vitro experiments.World J Gastrointest Oncol. 2024 Aug 15;16(8):3635-3650. doi: 10.4251/wjgo.v16.i8.3635. World J Gastrointest Oncol. 2024. PMID: 39171177 Free PMC article.
-
Antidiabetic Properties of Curcumin: Insights on New Mechanisms.Adv Exp Med Biol. 2021;1291:151-164. doi: 10.1007/978-3-030-56153-6_9. Adv Exp Med Biol. 2021. PMID: 34331689 Review.
-
Phytochemicals with protective effects against acute pancreatitis: a review of recent literature.Pharm Biol. 2022 Dec;60(1):479-490. doi: 10.1080/13880209.2022.2039723. Pharm Biol. 2022. PMID: 35180016 Free PMC article.
-
Prussian blue nanozyme-mediated nanoscavenger ameliorates acute pancreatitis via inhibiting TLRs/NF-κB signaling pathway.Theranostics. 2021 Jan 1;11(7):3213-3228. doi: 10.7150/thno.52010. eCollection 2021. Theranostics. 2021. PMID: 33537083 Free PMC article.
References
-
- Andersson B., Appelgren B., Sjodin V. Acute pancreatitis--costs for healthcare and loss of production. Scand. J. Gastroenterol. 2013;48(12):1459–1465. - PubMed
-
- Bhatia M., Wong F.L., Cao Y. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5(2–3):132–144. - PubMed
-
- Dawra R., Saluja A.K. L-arginine-induced experimental acute pancreatitis. Pancreapedia: Exocrine Pancreas Knowledge Base. 2012
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

