Biological response and developmental toxicity of zebrafish embryo and larvae exposed to multi-walled carbon nanotubes with different dimension
- PMID: 31485519
- PMCID: PMC6716136
- DOI: 10.1016/j.heliyon.2019.e02308
Biological response and developmental toxicity of zebrafish embryo and larvae exposed to multi-walled carbon nanotubes with different dimension
Abstract
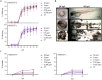
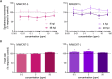
The development and use of nanomaterials are increasing significantly. Among nanomaterials, carbon nanotubes are of particular interest due to its distinctive physicochemical properties. This material composed of sheets of graphite has very high thermal conductivity, metallic-type electrical conductivity, stiffness, toughness and unique ability to bond to itself in an extended network with extraordinary strength. Its application in the industry is continuously growing, which could lead to the accumulation in the environment and a consequent impact on both humans and ecosystems. Considering that environmental systems are dynamic, it is difficult to predict the risks associated with the release of nanomaterials to the environment. Bioindicators are useful tools as primary signals of environmental risk, and their responses reveal the organism and ecosystem health. In the present study, we evaluated the impact of multi-walled carbon nanotubes with different dimensions and agglomeration pattern on zebrafish embryo and larvae; mainly, studies were focused on physiological and behavioral responses. In embryos, measurements were hatching rate, morphology changes, and viability. In larvae, locomotor activity, heart rate, innate inflammatory response, general and tissue-specific morphology were measured. MWCNT-S (short, wide and mostly dispersed) caused depression of the locomotor activity of larvae, indicating an alteration of the central nervous system, and depression of neutrophil migration activity. MWCNT-L (long, thin and agglomerated) caused malformations during larval development, a decrease of neutrophil migration and alteration of cardiac rhythm. Results obtained for both carbon nanotubes were different, highlighting the importance of dimensions of the same nanomaterial, and also the kind of agglomeration and shape adopted, for the toxic effects on organisms.
Keywords: Toxicology.
Figures






References
-
- Asharani P.V. Impact of multi-walled carbon nanotubes on aquatic species. J. Nanosci. Nanotechnol. 2008;8(7):3603–3609. - PubMed
-
- Cerrillo C. Ecotoxicity of multiwalled carbon nanotubes: standardization of the dispersion methods and concentration measurements. Environ. Toxicol. Chem. 2015;34(8):1854–1862. - PubMed
-
- Cimbaluk G.V. Evaluation of multiwalled carbon nanotubes toxicity in two fish species. Ecotoxicol. Environ. Saf. 2018;150:215–223. - PubMed
-
- Cheng J. Effect of carbon nanotubes on developing zebrafish (Danio rerio) embryos. Environ. Toxicol. Chem. 2007;26(4):708–716. - PubMed
LinkOut - more resources
Full Text Sources

