Co‑culturing with hypoxia pre‑conditioned mesenchymal stem cells as a new strategy for the prevention of irradiation‑induced fibroblast‑to‑myofibroblast transition
- PMID: 31485596
- PMCID: PMC6775806
- DOI: 10.3892/or.2019.7293
Co‑culturing with hypoxia pre‑conditioned mesenchymal stem cells as a new strategy for the prevention of irradiation‑induced fibroblast‑to‑myofibroblast transition
Abstract
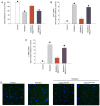
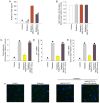
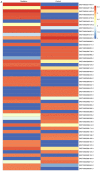
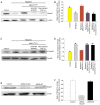
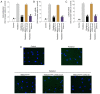
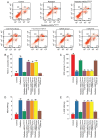
Cardiac fibrosis is a pathological consequence of radiation‑induced fibroblast proliferation and fibroblast‑to‑myofibroblast transition (FMT). Mesenchymal stem cell (MSC) transplantation has been revealed to be an effective treatment strategy to inhibit cardiac fibrosis. We identified a novel MSC‑driven mechanism that inhibited cardiac fibrosis, via the regulation of multiple fibrogenic pathways. Hypoxia pre‑conditioned MSCs (MSCsHypoxia) were co‑cultured with fibroblasts using a Transwell system. Radiation‑induced fibroblast proliferation was assessed using an MTT assay, and FMT was confirmed by assessing the mRNA levels of various markers of fibrosis, including type I collagen (Col1) and alpha smooth muscle actin (α‑SMA). α‑SMA expression was also confirmed via immunocytochemistry. The expression levels of Smad7 and Smad3 were detected by western blotting, and Smad7 was silenced using small interfering RNAs. The levels of oxidative stress following radiation were assessed by the detection of reactive oxygen species (ROS) and the activity of superoxide dismutase (SOD), malondialdehyde (MDA), and 4‑hydroxynonenal (HNE). It was revealed that co‑culturing with MSCsHypoxia could inhibit fibroblast proliferation and FMT. In addition, the present results indicated that MSCs are necessary and sufficient for the inhibition of fibroblast proliferation and FMT by functionally targeting TGF‑β1/Smad7/Smad3 signaling via the release of hepatocyte growth factor (HGF). Furthermore, it was observed that MSCs inhibited fibrosis by modulating oxidative stress. Co‑culturing with MSCsHypoxia alleviated fibroblast proliferation and FMT via the TGF‑β1/Smad7/Smad3 pathway. MSCs may represent a novel therapeutic approach for the treatment of radiation‑related cardiac fibrosis.
Figures

References
-
- Swerdlow AJ, Higgins CD, Smith P, Cunningham D, Hancock BW, Horwich A, Hoskin PJ, Lister A, Radford JA, Rohatiner AZ, Linch DC. Myocardial infarction mortality risk after treatment for Hodgkin disease: A collaborative British cohort study. J Natl Cancer Inst. 2007;99:206–214. doi: 10.1093/jnci/djk029. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

