ROCK1 promotes migration and invasion of non‑small‑cell lung cancer cells through the PTEN/PI3K/FAK pathway
- PMID: 31485605
- PMCID: PMC6741846
- DOI: 10.3892/ijo.2019.4864
ROCK1 promotes migration and invasion of non‑small‑cell lung cancer cells through the PTEN/PI3K/FAK pathway
Abstract
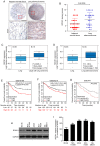
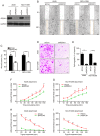
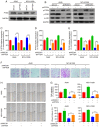
Rho‑associated protein kinase 1 (ROCK1), a member of the ROCK family, serves an important function in cell migration and invasion in neoplasms. ROCK1 has been found to be overexpressed in several types of cancers. However, the role of ROCK1 in non‑small‑cell lung cancer (NSCLC) is poorly understood. In the present study, ROCK1 was found to be overexpressed in NSCLC cells and tissues, and it was associated with poor survival of NSCLC patients. Subsequently, ROCK1 knockdown NSCLC cell lines were established using shRNA. ROCK1 knockdown significantly reduced the migration and invasion ability in the cell monolayer scratching and Transwell assays. ROCK1 knockdown was also found to markedly inhibit cell adhesion ability. Moreover, the phosphorylation of focal adhesion kinase (FAK) was inhibited by ROCK1 knockdown, reducing NSCLC cell migration and invasion ability. This mechanistic study revealed that ROCK1 significantly enhanced cell migration and invasion by inhibiting the phosphatase and tensin homolog (PTEN)/phosphoinositide 3‑kinase (PI3K)/FAK pathway. More importantly, the interruption of the PTEN/PI3K/FAK pathway markedly rescued the inhibition of cell migration and invasion mediated by ROCK1 knockdown. Taken together, these results suggest a novel role for ROCK1 in cell migration and invasion by inhibiting cell adhesion ability, and indicate that ROCK1 may be of value as a therapeutic target for the treatment of NSCLC.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

