Rsf‑1 regulates malignant melanoma cell viability and chemoresistance via NF‑κB/Bcl‑2 signaling
- PMID: 31485613
- PMCID: PMC6755232
- DOI: 10.3892/mmr.2019.10610
Rsf‑1 regulates malignant melanoma cell viability and chemoresistance via NF‑κB/Bcl‑2 signaling
Abstract
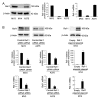
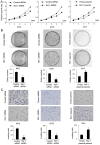
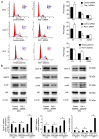
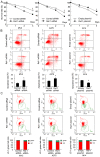
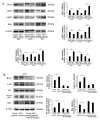
Remodeling and spacing factor 1 (Rsf‑1) has been reported as overexpressed in numerous cancers; however, its expression, biological functions and mechanisms in malignant melanoma remain unknown. In the present study, the expression of Rsf‑1 was investigated in 50 cases of malignant melanoma samples using immunohistochemistry. The results revealed that Rsf‑1 expression was elevated in 38% of specimens. MTT, colony formation, Transwell and flow cytometry assays were performed to investigate the functions of Rsf‑1. Knockdown of Rsf‑1 in the MV3 and A375 melanoma cell lines decreased the viability, invasion and cell cycle transition of cells. Conversely, overexpression of Rsf‑1 in M14 cells with low endogenous Rsf‑1 expression induced opposing effects. Further analysis revealed that Rsf‑1 knockdown decreased matrix metalloproteinase‑2, cyclin E and phosphorylated‑IκB expression. Additionally, Rsf‑1 depletion reduced cisplatin resistance and significantly increased the cisplatin‑associated apoptotic rate, whereas Rsf‑1 overexpression exhibited opposing effects. Rsf‑1 also maintained the mitochondrial membrane potential following cisplatin treatment. Analysis of apoptosis‑associated proteins revealed that Rsf‑1 positively regulated B‑cell lymphoma 2 (Bcl‑2), cellular inhibitor of apoptosis 1 (cIAP1) and cIAP2, and downregulated Bcl‑2‑associated X protein expression. Nuclear factor κ‑light‑chain‑enhancer of activated B‑cells (NF‑κB) inhibition reversed the effects of Rsf‑1 on Bcl‑2. In conclusion, Rsf‑1 was overexpressed in malignant melanoma and may contribute to the malignant behaviors of melanoma cells, possibly via the regulation of NF‑κB signaling. Therefore, Rsf‑1 may be a potential therapeutic target in the treatment of malignant melanoma.
Figures

References
-
- Merrill SJ, Subramanian M, Godar DE. Worldwide cutaneous malignant melanoma incidences analyzed by sex, age, and skin type over time (1955–2007): Is HPV infection of androgenic hair follicular melanocytes a risk factor for developing melanoma exclusively in people of European-ancestry? Dermatoendocrinol. 2016;8:e1215391. doi: 10.1080/19381980.2016.1215391. - DOI - PMC - PubMed
-
- Li H, Pedersen L, Nørgaard M, Ulrichsen SP, Thygesen SK, Nelson JJ. The occurrence of non-melanoma malignant skin lesions and non-cutaneous squamous-cell carcinoma among metastatic melanoma patients: An observational cohort study in Denmark. BMC Cancer. 2016;16:295. doi: 10.1186/s12885-016-2315-0. - DOI - PMC - PubMed
-
- Peterson M, Albertini MR, Remington P. Remington, incidence, survival, and mortality of malignant cutaneous melanoma in wisconsin, 1995–2011. WMJ. 2015;114:196–201. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

