Citrus unshiu peel suppress the metastatic potential of murine melanoma B16F10 cells in vitro and in vivo
- PMID: 31486124
- PMCID: PMC6916627
- DOI: 10.1002/ptr.6497
Citrus unshiu peel suppress the metastatic potential of murine melanoma B16F10 cells in vitro and in vivo
Abstract
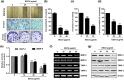
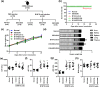
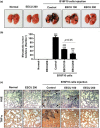
The peel of Citrus unshiu Marcow. fruits (CU) has long been used as a traditional medicine that has therapeutic effects against pathogenic diseases, including asthma, vomiting, dyspepsia, blood circulation disorders, and various types of cancer. In this study, we investigated the effect of CU peel on metastatic melanoma, a highly aggressive skin cancer, in B16F10 melanoma cells, and in B16F10 cells inoculated-C57BL/6 mice. Our results show that ethanol extracts of CU (EECU) inhibited cell growth and increased the apoptotic cells in B16F10 cells. EECU also stimulated the induction of mitochondria-mediated intrinsic pathway, with reduced mitochondrial membrane potential and increased generation of intracellular reactive oxygen species. Furthermore, EECU suppressed the migration, invasion, and colony formation of B16F10 cells. In addition, the oral administration of EECU reduced serum lactate dehydrogenase activity without weight loss, hepatotoxicity, nor nephrotoxicity in B16F10 cell-inoculated mice. Moreover, EECU markedly suppressed lung hypertrophy, the number and expression of metastatic tumor nodules, and the expression of inflammatory tumor necrosis factor-alpha in lung tissue. In conclusion, our findings suggest that the inhibitory effect of EECU on the metastasis of melanoma indicates that it may be regarded as a potential therapeutic herbal drug for melanoma.
Keywords: B16F10 cells; Citrus unshiu (CU); apoptosis; melanoma; metastasis.
© 2019 The Authors. Phytotherapy Research published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
-
- Ahn, K. I. , Choi, E. O. , Kwon, D. H. , HwangBo, H. , Kim, M. Y. , Kim, H. J. , … Choi, Y. H. (2017). Induction of apoptosis by ethanol extract of Citrus unshiu Markovich peel in human bladder cancer T24 cells through ROS‐mediated inactivation of the PI3K/Akt pathway. Bioscience Trends, 11(5), 565–573. 10.5582/bst.2017.01218 - DOI - PubMed
-
- Aroui, S. , Aouey, B. , Chtourou, Y. , Meunier, A. C. , Fetoui, H. , & Kenani, A. (2015). Naringin suppresses cell metastasis and the expression of matrix metalloproteinases (MMP‐2 and MMP‐9) via the inhibition of ERK‐P38‐JNK signaling pathway in human glioblastoma. Chemico‐Biological Interactions, 244, 195–203. 10.1016/j.cbi.2015.12.011 - DOI - PubMed
-
- Aroui, S. , Najiaoui, F. , Chtourou, Y. , Meunier, A. C. , Laaijimi, A. , Kenani, A. , … Fetoui, H. (2016). Naringin inhibits the invasion and migration of human glioblastoma cell via downregulation of MMP‐2 and MMP‐9 expression and inactivation of p38 signaling pathway. Tumour Biology, 37(3), 3831–3839. 10.1007/s13277-015-4230-4 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

