Dynorphin-based "release on demand" gene therapy for drug-resistant temporal lobe epilepsy
- PMID: 31486590
- PMCID: PMC6783645
- DOI: 10.15252/emmm.201809963
Dynorphin-based "release on demand" gene therapy for drug-resistant temporal lobe epilepsy
Abstract
Focal epilepsy represents one of the most common chronic CNS diseases. The high incidence of drug resistance, devastating comorbidities, and insufficient responsiveness to surgery pose unmet medical challenges. In the quest of novel, disease-modifying treatment strategies of neuropeptides represent promising candidates. Here, we provide the "proof of concept" that gene therapy by adeno-associated virus (AAV) vector transduction of preprodynorphin into the epileptogenic focus of well-accepted mouse and rat models for temporal lobe epilepsy leads to suppression of seizures over months. The debilitating long-term decline of spatial learning and memory is prevented. In human hippocampal slices obtained from epilepsy surgery, dynorphins suppressed seizure-like activity, suggestive of a high potential for clinical translation. AAV-delivered preprodynorphin expression is focally and neuronally restricted and release is dependent on high-frequency stimulation, as it occurs at the onset of seizures. The novel format of "release on demand" dynorphin delivery is viewed as a key to prevent habituation and to minimize the risk of adverse effects, leading to long-term suppression of seizures and of their devastating sequel.
Keywords: adeno-associated virus; learning; memory; neuropeptide; seizure.
© 2019 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
A PCT application of the described technology is pending. R.H. is inventor of a patent related to rAAV technology and owns equity in a company commercializing AAV for gene therapy.
Figures

- A
Displayed (sc)AAV2‐based vector backbones were packaged in AAV serotype 1 capsids. AAV‐ITRs are displayed in gray, and ΔITR refers to the mutated ITR version of scAAVs. The transgene of AAV‐pDyn is a codon‐optimized version of the full‐length human preprodynorphin cDNA enhanced by a WPRE element. Control vectors carry either a truncated, non‐functional version of the enhanced GFP gene (AAV‐ΔGFP), or its functional counterpart (AAV‐eGFP; not displayed).
- B
Kainic acid mouse model of TLE: Daily EEG recordings obtained from the epileptogenic focus starting from 1 month after KA injection and spanning the period from 2 days before to 7 days after AAV‐pDyn delivery (2 × 109 gp).
- C
Higher time resolution of the indicated section of the Day +1 in (B), representing a hippocampal paroxysmal discharge (HPD). A generalized seizure is depicted in Fig EV1.
- D, E
The characteristic EEG features of this model, secondary generalized seizures (bars) and HPDs (lines), were reduced in number and in duration by AAV‐pDyn (red; n = 3 (from day 60); 7 (till day 30) per time interval), but not by AAV‐ΔGFP or after sham treatment (blue; n = 4 (day 90); 5 (day 30 and 60); and 6 (before day 30), per time interval). The relatively high variability of seizure frequencies and duration is typical for this model and reflects the findings in human mTLE. Some animals could not be recorded for the entire period due to loss of implant. **P < 0.01; effect of treatment on HPDs; ## P < 0.01; effect of treatment on generalized seizures; analyzed by two‐way ANOVA with Bonferroni correction for both number and time.
- F
Injection of norBNI (20 mg/kg; i.p.) results in a transient reappearance of HPDs immediately and 24 h after application. One week after norBNI application (washout), suppression of HPDs was re‐established. Data obtained from 4 epileptic animals before (black) and after (red) AAV‐pDyn delivery (2 × 109 gp) are depicted. *P < 0.05; **P < 0.01; one‐way ANOVA for repeated measures with Friedman post hoc test.
- G
EEG recordings obtained from the ipsilateral dorsal hippocampus of rats after electrical self‐sustained status epilepticus (SSSE) before (black) and after (red) AAV‐pDyn delivery (4x109 gp) are depicted. Spike trains with a frequency of at least 2.5 Hz induced by SSSE were markedly reduced by AAV‐pDyn (n = 4). *P < 0.05 one‐way ANOVA for repeated measures with Friedman post hoc test.

- A
EEG trace of a generalized seizure in a KA‐treated mouse; from top to bottom contralateral hippocampus, contralateral motor cortex and ipsilateral hippocampus.
- B
Blow‐up of the EEG trace from the ipsilateral hippocampus in panel (A).

- A–I
Spatial learning and memory were tested on the Barnes maze. Quadrant 1 (Q1) contains the target hole (red; A). Unilateral injection of AAV‐pDyn (B) or AAV‐eGFP (C) into naïve young adult mice (12 weeks age) did not influence the performance as compared to naïve controls (D) when tested 4 weeks after AAV injection. Mice treated 2 weeks after KA with AAV‐pDyn (E, H) performed equally to age‐matched naïve controls (D, G) 2 weeks (E) and 5.5 months (H) after treatment. By contrast, animals treated 2 weeks after KA with AAV‐ΔGFP (F, I) gradually lost this ability. Two‐way ANOVA revealed significance between AAV‐ΔGFP‐ and AAV‐pDyn‐treated groups for interaction 2 weeks (P = 0.0349) and 5.5 months (P = 0.0311) after AAV, respectively, at each time interval and quadrant (P < 0.0001) 2 weeks after AAV. Comparison of AAV‐pDyn injected with naïve animals revealed no differences.
- J–O
Epileptic mice, which were not able to learn the Barnes maze task 1 month after KA (J, M), AAV‐pDyn application restored spatial memory 1 (K) and 2 months (L) after treatment. Treatment with AAV‐ΔGFP did not result in improved memory (N, O). Two‐way ANOVA revealed significance for interaction (P = 0.0049) and quadrant (< 0.0001) comparing AAV‐ΔGFP with AAV‐pDyn‐treated animals at the later time interval.
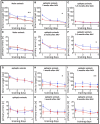
- A–F
Unilateral injection of AAV‐pDyn (red) or AAV‐ΔGFP (blue) into naïve young adult mice compared with naive controls (black) is depicted in (A) and (D). Interestingly, AAV‐ΔGFP‐injected mice took longer to find the hole. Noteworthy, this had no impact on memory retrieval (Fig 2A–C). Mice treated 2 weeks after KA with AAV‐pDyn (red) or AAV‐ΔGFP (blue) (B, C, E, F) performed differently 2 weeks after vector application (B, E) in respect to time needed to target and 5.5 months (C, F) after treatment in respect to primary errors. The overall reduction in time needed to target is most probably due to the repeated testing of animals on the Barnes maze. The target hole was repositioned in each round, but the mice were familiar with the test per se.
- G–L
A person not familiar with the mice assigned epileptic animals into two groups before testing on Barnes maze. No differences were observed between the two groups before vector treatment (G, J). By contrast, AAV‐pDyn (red)‐treated animals reached the target significantly faster than AAV‐ΔGFP (blue) 1 and 2 months after treatment (H, I). Primary errors did not differ (K, L).

- A–F
Double‐immunofluorescence labeling is depicted for pDyn and NeuN (A–C) or GFAP (D–F) in the ipsilateral dentate gyrus of KA‐treated and AAV‐pDyn‐injected mice. Enlarged view in (F) represents 15 × 30 μm.
- G
Mature Dyn B content (measured by a Dyn B‐specific EIA) in the dorsal hippocampus of mice treated with KA (blue symbols) or KA and AAV‐pDyn (red symbols) 1.5 (open symbols; n = 6) and 6 (filled symbols; n = 3) months after vector treatment. Naïve animals were age‐matched to the 1.5 months after AAV group. iH stands for ipsilateral hippocampus and cH for contralateral hippocampus. *P < 0.05; paired t‐test was used for comparison of ipsi‐ and contralateral hippocampi. Two‐way ANOVA was used to compare Dyn levels between the early and late time interval.
- H
Mature Dyn B content in the CSF of mice treated with KA (blue symbols) or KA and AAV‐pDyn (red symbols) 1.5 (open symbols; n = 6) and 7 (filled symbols; n = 4) months after vector treatment. **P < 0.01; one‐way ANOVA with Dunnett post hoc test
- I
The release of fully processed, mature Dyn B under different stimulation conditions was analyzed in microdialysates collected from the hippocampus of pDyn‐deficient (KO) animals 2 weeks after injection of AAV‐pDyn. Three baseline samples (BL; 25 min each) were collected. This was followed by 25 min low‐frequency stimulation (LF), 25 min baseline, and 25 min high‐frequency stimulation (HF; I).
- J
The microdialysis probe (red cross) was placed in the dentate gyrus, and the stimulation electrode (black cross) in the entorhinal cortex.
- K
Dyn B was quantified by EIA in the dialysate collected during different stimulation intensities. The red line represents the detection limit of the EIA. *P < 0.05; n = 4; one‐way ANOVA with Tukey post hoc test.

- A–D
Immunohistochemistry was performed on PFA‐fixed 40‐μm sections obtained from a pDyn knockout mouse unilaterally injected with 2 × 109 gp of AAV‐pDyn 3 weeks before. The ipsilateral hippocampus (A) displays strong immunoreactivity in the terminal field of mossy fibers and the polymorph cell layer, indicative for expression in granule cells and localization in granule cell axons. Strong immunoreactivity was also observed in the inner molecular layer in both hippocampi (A, B). This region is strongly innervated by mossy cells, located in the hilus and forming strong projections also contralaterally. Several non‐principal neurons were labeled in the ipsilateral polymorph cell layer (C). These cells may represent different types of GABAergic interneurons beside the glutamatergic mossy cells. Labeling in the molecular layer is dense in the supergranular band but faint in the middle and outer layers. Some well‐labeled filaments (presumably fibers due to their varicose structure) are visible (D). The diffuse light labeling in the ipsilateral but not contralateral molecular layer might be due to Dyn stored in dendrites, as suggested by some studies, or represent axon terminals of GABAergic interneurons. Scale bars represent 500 μm (A) and (B); 100 μm (C); and 50 μm (D). Box in (A) delineates the section enlarged in (C). Box in (C) delineates the section enlarged in (D). Immunohistochemistry was performed using rabbit anti‐human dynorphin B antibodies (Serotec AHP377; 1: 1,000) and goat anti‐rabbit secondary antibodies coupled to horseradish peroxidase (Dako P 0448; 1: 400) followed by 3,3′‐diaminobenzidine staining.
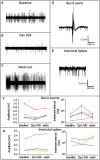
- A–C
Representative traces under conditions of increased KCl (A), together with Dyn A/B (B), or under co‐application of Dyn A/B with 5′‐GNTI (C). * In (A, B) indicate the time interval shown in (D, E).
- D, E
Burst events (D) and interictal spikes (E) were recorded from area CA1.
- F, G
Treatment with Dyn A/B markedly reduced the amplitude and number of bursts (F) but not of interictal spikes (G). Each color represents the last 5 min of each application for one slice of 4 individual patients. Data sets for each patient represent mean ± SEM for amplitude or absolute number of events. Two‐way ANOVA for repeated measurements revealed high significance not only for interaction (P < 0.0001) and amplitude (P < 0.0001) or interevent intervals (P < 0.0001), but also for the factor patient (P < 0.0001). Therefore, careful interpretation of the raw data is considered as preferred method. For detailed data, see Table EV1.
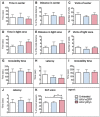
- A–K
Naive mice treated with either AAV‐eGFP (blue columns) or AAV‐pDyn (red columns) were tested in the open‐field (A–C), light–dark (D–F), forced swim (G, H) tail suspension (I, J), and spontaneous alteration (K) test. No differences were observed in anxiety (A–F) or stress‐coping (G–J) behavior. AAV‐pDyn‐injected animals displayed a higher ratio of correct arm entries (K). **P < 0.01, one‐way ANOVA. Data represent mean ± SD of n = 6 per group.
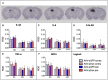
- A
In situ hybridization for AAV‐derived pDyn mRNA at 10 weeks post‐AAV‐pDyn delivery in the left dorsal hippocampus is depicted for a single brain at five levels between 1.1 (left) and 2.3 (right) mm from bregma. The probe specifically detects the codon‐optimized sequence; therefore, the contralateral side represents a control devoid of the respective mRNA.
- B–F
At 10 weeks post‐AAV‐pDyn delivery, markers of inflammation were analyzed as follows: Relative optical densities (ROD) were evaluated from film autoradiographs after in situ hybridization for mRNAs of inflammatory markers (interleukin‐1β (B), interleukin‐6 (C), interleukin‐1a receptor agonist (D), tumor necrosis factor‐α (E), and nitric oxide synthase‐1 (F)) in 3 principal sub‐regions of the hippocampus in AAV‐pDyn (n = 7; red)‐ or AAV‐eGFP (n = 5; blue)‐injected animals. GC = granule cell layer; CA = cornu ammonis. Data represent mean ± standard deviation.

Comment in
-
Is On-Demand Dynorphin Destined to Be in Demand to Decrease Seizures?Epilepsy Curr. 2020 Sep 10;21(1):48-50. doi: 10.1177/1535759720951791. eCollection 2021 Jan-Feb. Epilepsy Curr. 2020. PMID: 34025273 Free PMC article. No abstract available.
References
-
- Bergey GK (2013) Neurostimulation in the treatment of epilepsy. Exp Neurol 244: 87–95 - PubMed
-
- Bilkei‐Gorzo A, Racz I, Michel K, Mauer D, Zimmer A, Klingmuller D, Zimmer A (2008) Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology 33: 425–436 - PubMed
-
- van Diessen E, Hanemaaijer JI, Otte WM, Zelmann R, Jacobs J, Jansen FE, Dubeau F, Stam CJ, Gotman J, Zijlmans M (2013) Are high frequency oscillations associated with altered network topology in partial epilepsy? NeuroImage 82: 564–573 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

