Pharmacological characterization of a homomeric nicotinic acetylcholine receptor formed by Ancylostoma caninum ACR-16
- PMID: 31486912
- PMCID: PMC7869652
- DOI: 10.1007/s10158-019-0231-0
Pharmacological characterization of a homomeric nicotinic acetylcholine receptor formed by Ancylostoma caninum ACR-16
Abstract
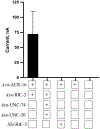
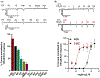
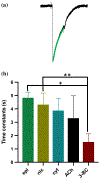
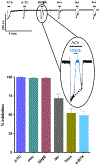
Parasitic nematode infections are treated using anthelmintic drugs, some of which target nicotinic acetylcholine receptors (nAChRs) located in different parasite tissues. The limited arsenal of anthelmintic agents and the prevalence of drug resistance imply that future defense against parasitic infections will depend on the discovery of novel targets and therapeutics. Previous studies have suggested that Ascaris suum ACR-16 nAChRs are a suitable target for the development of antinematodal drugs. In this study, we characterized the pharmacology of the Ancylostoma caninum ACR-16 receptor using two-electrode voltage-clamp electrophysiology. This technique allowed us to study the effects of cholinergic agonists and antagonists on the nematode nAChRs expressed in Xenopus laevis oocytes. Aca-ACR-16 was not sensitive to many of the existing cholinomimetic anthelmintics (levamisole, oxantel, pyrantel, and tribendimidine). 3-Bromocytisine was the most potent agonist (> 130% of the control acetylcholine current) on the Aca-ACR-16 nAChR but, unlike Asu-ACR-16, oxantel did not activate the receptor. The mean time constants of desensitization for agonists on Aca-ACR-16 were longer than the rates observed in Asu-ACR-16. In contrast to Asu-ACR-16, the A. caninum receptor was completely inhibited by DHβE and moderately inhibited by α-BTX. In conclusion, we have successfully reconstituted a fully functional homomeric nAChR, ACR-16, from A. caninum, a model for human hookworm infections. The pharmacology of the receptor is distinct from levamisole-sensitive nematode receptors. The ACR-16 homologue also displayed some pharmacological differences from Asu-ACR-16. Hence, A. caninum ACR-16 may be a valid target site for the development of anthelmintics against hookworm infections.
Keywords: Aca-ACR-16; Anthelmintic; Hookworms; Xenopus oocyte; nAChR.
Conflict of interest statement
Figures

Similar articles
-
Pharmacological profile of Ascaris suum ACR-16, a new homomeric nicotinic acetylcholine receptor widely distributed in Ascaris tissues.Br J Pharmacol. 2016 Aug;173(16):2463-77. doi: 10.1111/bph.13524. Epub 2016 Jul 18. Br J Pharmacol. 2016. PMID: 27238203 Free PMC article.
-
The cholinomimetic morantel as an open channel blocker of the Ascaris suum ACR-16 nAChR.Invert Neurosci. 2016 Dec;16(4):10. doi: 10.1007/s10158-016-0193-4. Epub 2016 Dec 19. Invert Neurosci. 2016. PMID: 27995347
-
The nicotinic acetylcholine receptors of the parasitic nematode Ascaris suum: formation of two distinct drug targets by varying the relative expression levels of two subunits.PLoS Pathog. 2009 Jul;5(7):e1000517. doi: 10.1371/journal.ppat.1000517. Epub 2009 Jul 17. PLoS Pathog. 2009. PMID: 19609360 Free PMC article.
-
Nicotinic acetylcholine receptors: a comparison of the nAChRs of Caenorhabditis elegans and parasitic nematodes.Parasitol Int. 2013 Dec;62(6):606-15. doi: 10.1016/j.parint.2013.03.004. Epub 2013 Mar 15. Parasitol Int. 2013. PMID: 23500392 Review.
-
Compte rendu The canine hookworm Ancylostoma caninum: A novel threat for anthelmintic resistance in Canada.Can Vet J. 2023 Apr;64(4):372-378. Can Vet J. 2023. PMID: 37008647 Free PMC article. Review.
Cited by
-
Two residues determine nicotinic acetylcholine receptor requirement for RIC-3.Protein Sci. 2023 Sep;32(9):e4718. doi: 10.1002/pro.4718. Protein Sci. 2023. PMID: 37417463 Free PMC article.
-
Caenorhabditis elegans muscle Cys-loop receptors as novel targets of terpenoids with potential anthelmintic activity.PLoS Negl Trop Dis. 2019 Nov 25;13(11):e0007895. doi: 10.1371/journal.pntd.0007895. eCollection 2019 Nov. PLoS Negl Trop Dis. 2019. PMID: 31765374 Free PMC article.
-
Nodulisporic acid produces direct activation and positive allosteric modulation of AVR-14B, a glutamate-gated chloride channel from adult Brugia malayi.Proc Natl Acad Sci U S A. 2022 Aug 23;119(34):e2111932119. doi: 10.1073/pnas.2111932119. Epub 2022 Aug 15. Proc Natl Acad Sci U S A. 2022. PMID: 35969762 Free PMC article.
-
Reconstitution of an N-AChR from Brugia malayi, an evolved change in acetylcholine receptor accessory protein requirements in filarial parasites.PLoS Pathog. 2022 Nov 14;18(11):e1010962. doi: 10.1371/journal.ppat.1010962. eCollection 2022 Nov. PLoS Pathog. 2022. PMID: 36374934 Free PMC article.
-
A Functional Comparison of Homopentameric Nicotinic Acetylcholine Receptors (ACR-16) Receptors From Necator americanus and Ancylostoma ceylanicum.Front Mol Neurosci. 2020 Nov 26;13:601102. doi: 10.3389/fnmol.2020.601102. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33324163 Free PMC article.
References
-
- Albonico M, Smith PG, Ercole E, Hall A, Chwaya HM, Alawi KS et al. (1995) Rate of reinfection with intestinal nematodes after treatment of children with mebendazole or albendazole in a highly endemic area. Trans R Soc Trop Med Hyg 89(5):538–541 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

