Serum sodium and intracranial pressure changes after desmopressin therapy in severe traumatic brain injury patients: a multi-centre cohort study
- PMID: 31486921
- PMCID: PMC6728106
- DOI: 10.1186/s13613-019-0574-z
Serum sodium and intracranial pressure changes after desmopressin therapy in severe traumatic brain injury patients: a multi-centre cohort study
Erratum in
-
Correction to: Serum sodium and intracranial pressure changes after desmopressin therapy in severe traumatic brain injury patients: a multi-centre cohort study.Ann Intensive Care. 2019 Dec 4;9(1):136. doi: 10.1186/s13613-019-0610-z. Ann Intensive Care. 2019. PMID: 31802308 Free PMC article.
Abstract
Background: In traumatic brain injury (TBI) patients desmopressin administration may induce rapid decreases in serum sodium and increase intracranial pressure (ICP).
Aim: In an international multi-centre study, we aimed to report changes in serum sodium and ICP after desmopressin administration in TBI patients.
Methods: We obtained data from 14 neurotrauma ICUs in Europe, Australia and UK for severe TBI patients (GCS ≤ 8) requiring ICP monitoring. We identified patients who received any desmopressin and recorded daily dose, 6-hourly serum sodium, and 6-hourly ICP.
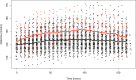
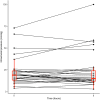
Results: We studied 262 severe TBI patients. Of these, 39 patients (14.9%) received desmopressin. Median length of treatment with desmopressin was 1 [1-3] day and daily intravenous dose varied between centres from 0.125 to 10 mcg. The median hourly rate of decrease in serum sodium was low (- 0.1 [- 0.2 to 0.0] mmol/L/h) with a median period of decrease of 36 h. The proportion of 6-h periods in which the rate of natremia correction exceeded 0.5 mmol/L/h or 1 mmol/L/h was low, at 8% and 3%, respectively, and ICPs remained stable. After adjusting for IMPACT score and injury severity score, desmopressin administration was independently associated with increased 60-day mortality [HR of 1.83 (1.05-3.24) (p = 0.03)].
Conclusions: In severe TBI, desmopressin administration, potentially representing instances of diabetes insipidus is common and is independently associated with increased mortality. Desmopressin doses vary markedly among ICUs; however, the associated decrease in natremia rarely exceeds recommended rates and median ICP values remain unchanged. These findings support the notion that desmopressin therapy is safe.
Keywords: Desmopressin; Diabetes insipidus; Natremia; Sodium; Traumatic brain injury.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Variability in Serum Sodium Concentration and Prognostic Significance in Severe Traumatic Brain Injury: A Multicenter Observational Study.Neurocrit Care. 2021 Jun;34(3):899-907. doi: 10.1007/s12028-020-01118-8. Epub 2020 Oct 2. Neurocrit Care. 2021. PMID: 33009658
-
The effect of continuous hypertonic saline infusion and hypernatremia on mortality in patients with severe traumatic brain injury: a retrospective cohort study.Can J Anaesth. 2016 Jun;63(6):664-73. doi: 10.1007/s12630-016-0633-y. Epub 2016 Mar 30. Can J Anaesth. 2016. PMID: 27030131 English.
-
Hypertonic saline reduces cumulative and daily intracranial pressure burdens after severe traumatic brain injury.J Neurosurg. 2015 Jan;122(1):202-10. doi: 10.3171/2014.10.JNS132545. J Neurosurg. 2015. PMID: 25380107 Clinical Trial.
-
Intracranial pressure monitoring among children with severe traumatic brain injury.J Neurosurg Pediatr. 2015 Nov;16(5):523-532. doi: 10.3171/2015.3.PEDS14507. Epub 2015 Aug 14. J Neurosurg Pediatr. 2015. PMID: 26273741
-
Intracranial pressure monitoring and inpatient mortality in severe traumatic brain injury: A propensity score-matched analysis.J Trauma Acute Care Surg. 2015 Mar;78(3):492-501; discussion 501-2. doi: 10.1097/TA.0000000000000559. J Trauma Acute Care Surg. 2015. PMID: 25710418
Cited by
-
Correction to: Serum sodium and intracranial pressure changes after desmopressin therapy in severe traumatic brain injury patients: a multi-centre cohort study.Ann Intensive Care. 2019 Dec 4;9(1):136. doi: 10.1186/s13613-019-0610-z. Ann Intensive Care. 2019. PMID: 31802308 Free PMC article.
-
Relationship Between the First Undisturbed Sleep Period and Diuretic and Urine Storage States of the First Void After Sleep.Neurourol Urodyn. 2025 Apr;44(4):736-742. doi: 10.1002/nau.70008. Epub 2025 Feb 12. Neurourol Urodyn. 2025. PMID: 39935423 Free PMC article.
References
-
- Alharfi IM, Stewart TC, Foster J, Morrison GC, Fraser DD. Central diabetes insipidus in pediatric severe traumatic brain injury. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2013;14:203–209. - PubMed
LinkOut - more resources
Full Text Sources

