Development and validation of the Axillary Sweating Daily Diary: a patient-reported outcome measure to assess axillary sweating severity
- PMID: 31486951
- PMCID: PMC6728105
- DOI: 10.1186/s41687-019-0148-8
Development and validation of the Axillary Sweating Daily Diary: a patient-reported outcome measure to assess axillary sweating severity
Abstract
Background: Hyperhidrosis is estimated to affect ~ 4.8% of the US population, and most patients experience a negative psychological impact. Here, we describe development and psychometric evaluation of a patient-reported outcome (PRO) measure to assess severity of axillary hyperhidrosis in clinical trials that meets current U.S. regulatory standards to support product approvals.
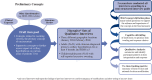
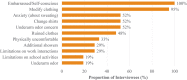
Methods: Three rounds of hybrid concept-elicitation/cognitive-debriefing qualitative interviews were conducted in adults with clinician-diagnosed primary axillary hyperhidrosis, followed by similar interviews in children/adolescents. The draft measure included diary items for presence, severity, impact and bothersomeness (basis of the Axillary Sweating Daily Diary [ASDD]), exploratory weekly impact items, and a single-item Patient Global Impression of Change (PGIC). Phase 2 (adults only) and phase 3 (adults and children ≥9 years) clinical trial data were utilized to evaluate measurement properties of the resulting draft measure: floor/ceiling effects, nonresponse bias, test-retest reliability, construct validity, and responsiveness were assessed. The primary concept of interest was axillary sweating severity (ASDD Item 2); however, additional supportive concepts were explored to allow for development of a comprehensive hyperhidrosis measure.
Results: Twenty-nine patient interviews were conducted (N = 21 adult and N = 8 children/adolescents), resulting in the ASDD (4 items, patients ≥16y) and child-specific ASDD-C (2 items ≥9y to <16y), as well as 6 Weekly Impact items and the PGIC (patients ≥16y). No floor/ceiling effects or response biases were identified. Consistency between hypothesized and observed correlation patterns between ASDD/ASDD-C items and other efficacy measures supported construct validity. Intraclass correlation coefficients supported test-retest reliability (0.91-0.93; Item 2). Large effect sizes (- 2.2 to - 2.4) demonstrated that the ASDD/ASDD-C Item 2 could detect changes in hyperhidrosis severity, supporting the measure's responsiveness. Patients perceiving a moderate improvement in symptoms on the PGIC experienced an average 3.8-point improvement on ASDD axillary sweating severity (Item 2); thus, a 4-point responder threshold was defined as a clinically meaningful change.
Conclusions: Qualitative and quantitative evidence support the reliability and validity of the ASDD/ASDD-C and its use in the clinical evaluation of axillary hyperhidrosis treatments. Further evaluation of this measure in future research studies is warranted to demonstrate consistent performance across different axillary hyperhidrosis populations and in different study contexts.
Keywords: Axillary sweating daily diary (ASDD); Hyperhidrosis; Patient reported outcome; Sweating severity.
Conflict of interest statement
LN, DD, and SF: Employee of RTI Health Solutions. DMP: Consultant (honoraria): Brickell Biotech, Inc.; Biofrontera AG; Celgene; Dermira, Inc.; DUSA Pharmaceuticals, Inc.; LEO Pharma; Novartis; Promius Pharma, LLC; Regeneron Pharmaceuticals, Inc.; Sanofi; TheraVida, Inc.; Valeant Pharmaceuticals International, Inc. Advisory board (honoraria): Pfizer, Inc. Investigator (grants/research funding): Abbott Laboratories; Amgen, Inc.; Brickell Biotech, Inc.; Celgene; Dermavant Sciences; Eli Lilly and Company; LEO Pharma; Merck & Co, Inc.; Novartis; Novo Nordisk A/S; Ortho Dermatologics; Peplin, Inc.; Photocure ASA; Promius Pharma, LLC; Regeneron Pharmaceuticals, Inc.; Stiefel Laboratories; Valeant Pharmaceuticals International, Inc. Investigator (honoraria): LEO Pharma; Pfizer, Inc. DAG: Consultant and investigator: Dermira, Inc. AAH: Investigator (research funding): Dermira, Inc. (paid to the UTHealth McGovern Medical School, Houston). Advisory board (honoraria): Dermira, Inc. Research funding (all monies paid to the UTHealth McGovern Medical School, Houston): Allergan, Plc.; Amgen, Inc.; Cassiopea; Celgene; Dermavant Sciences; Eli Lilly and Company; Galderma S.A.; GSK, Plc.; LEO Pharma; Mayne Pharma; Medimetriks Pharmaceuticals; Novan, Inc.; Promius Pharma, LLC; Vanda Pharmaceuticals. Honoraria: Amgen; GSK, Plc.; Pfizer, Inc.; Valeant Pharmaceutics International, Inc. HH, JD, KKG, and DI: Employee and stockholder of Dermira, Inc.
Figures
Similar articles
-
Topical Glycopyrronium Tosylate for the Treatment of Primary Axillary Hyperhidrosis: Patient-Reported Outcomes from the ATMOS-1 and ATMOS-2 Phase III Randomized Controlled Trials.Am J Clin Dermatol. 2019 Feb;20(1):135-145. doi: 10.1007/s40257-018-0395-0. Am J Clin Dermatol. 2019. PMID: 30378087 Free PMC article. Clinical Trial.
-
Glycopyrronium tosylate in pediatric primary axillary hyperhidrosis: Post hoc analysis of efficacy and safety findings by age from two phase three randomized controlled trials.Pediatr Dermatol. 2019 Jan;36(1):89-99. doi: 10.1111/pde.13723. Epub 2018 Nov 19. Pediatr Dermatol. 2019. PMID: 30451318 Free PMC article. Clinical Trial.
-
The Hyperhidrosis Disease Severity Measure-Axillary: Conceptualization and Development of Item Content.J Drugs Dermatol. 2018 Jul 1;17(7):707-714. J Drugs Dermatol. 2018. PMID: 30005091
-
Efficacy and safety of topical glycopyrronium bromide in treating axillary hyperhidrosis: systematic review and meta-analysis.Sci Rep. 2024 Oct 19;14(1):24537. doi: 10.1038/s41598-024-74430-4. Sci Rep. 2024. PMID: 39424822 Free PMC article.
-
Understanding treatment burden in hemophilia: development and validation of the Hemophilia Treatment Experience Measure (Hemo-TEM).J Patient Rep Outcomes. 2023 Feb 23;7(1):17. doi: 10.1186/s41687-023-00550-6. J Patient Rep Outcomes. 2023. PMID: 36821002 Free PMC article. Review.
Cited by
-
Limited Systemic Exposure with Topical Glycopyrronium Tosylate in Primary Axillary Hyperhidrosis.Clin Pharmacokinet. 2021 May;60(5):665-676. doi: 10.1007/s40262-020-00975-y. Epub 2021 Jan 12. Clin Pharmacokinet. 2021. PMID: 33433785 Free PMC article. Clinical Trial.
-
A glycopyrronium bromide 1% cream for topical treatment of primary axillary hyperhidrosis: efficacy and safety results from a phase IIIa randomized controlled trial.Br J Dermatol. 2021 Aug;185(2):315-322. doi: 10.1111/bjd.19810. Epub 2021 Mar 2. Br J Dermatol. 2021. PMID: 33445205 Free PMC article. Clinical Trial.
-
Understanding data visualization techniques in qualitative studies used to develop and validate patient-reported outcome measures: a targeted literature review.Qual Life Res. 2025 Jul;34(7):2031-2048. doi: 10.1007/s11136-025-03964-5. Epub 2025 Apr 25. Qual Life Res. 2025. PMID: 40279025 Review.
-
Questionnaire-based epidemiological survey of primary focal hyperhidrosis and survey on current medical management of primary axillary hyperhidrosis in Japan.Arch Dermatol Res. 2023 Apr;315(3):409-417. doi: 10.1007/s00403-022-02365-9. Epub 2022 Jun 29. Arch Dermatol Res. 2023. PMID: 35768620 Free PMC article.
-
Topical glycopyrronium tosylate in Japanese patients with primary axillary hyperhidrosis: A randomized, double-blind, vehicle-controlled study.J Dermatol. 2022 Jan;49(1):86-94. doi: 10.1111/1346-8138.16188. Epub 2021 Oct 11. J Dermatol. 2022. PMID: 34636057 Free PMC article. Clinical Trial.
References
-
- Tetteh HA, Groth SS, Kast T, Whitson BA, Radosevich DM, Klopp AC, D'Cunha J, Maddaus MA, Andrade RS. Primary palmoplantar hyperhidrosis and thoracoscopic sympathectomy: A new objective assessment method. The Annals of Thoracic Surgery. 2009;87:267–274. doi: 10.1016/j.athoracsur.2008.10.028. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical