Heterogeneity of Pulmonary Stem Cells
- PMID: 31487021
- PMCID: PMC8421072
- DOI: 10.1007/978-3-030-24108-7_6
Heterogeneity of Pulmonary Stem Cells
Abstract
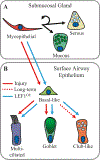
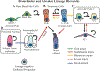
Epithelial stem cells reside within multiple regions of the lung where they renew various region-specific cells. In addition, there are multiple routes of regeneration after injury through built-in heterogeneity within stem cell populations and through a capacity for cellular plasticity among differentiated cells. These processes are important facets of respiratory tissue resiliency and organism survival. However, this regenerative capacity is not limitless, and repetitive or chronic injuries, environmental stresses, or underlying factors of disease may ultimately lead to or contribute to tissue remodeling and end-stage lung disease. This chapter will review stem cell heterogeneity among pulmonary epithelia in the lower respiratory system, discuss recent findings that may challenge long-held scientific paradigms, and identify several clinically relevant research opportunities for regenerative medicine.
Keywords: Alveolar type II cell; Basal stem cell; Chronic rejection; Club cell; Goblet cell; Ionocyte; Myoepithelial cell; Obliterative bronchiolitis; Stem cell; Submucosal gland.
Figures

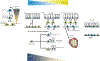

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

