Cross-sectional analysis of the Myasthenia Gravis Patient Registry: Disability and treatment
- PMID: 31487038
- PMCID: PMC6899582
- DOI: 10.1002/mus.26695
Cross-sectional analysis of the Myasthenia Gravis Patient Registry: Disability and treatment
Abstract
Introduction: The Myasthenia Gravis Patient Registry (MGR) is a voluntary, patient-submitted database dedicated to improve understanding of care/burden of myasthenia gravis (MG).
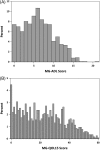
Methods: In this study we present analyses of baseline records through July 2017 (n = 1140) containing data on the MG-Activities of Daily Living (MG-ADL) and the MG 15-item Quality of Life (MG-QOL15) instruments, two validated scales assessing quality of life in MG patients at sign-up into the MGR.
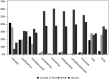
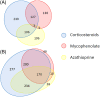
Results: Most registrants reported moderate to severe impairment of health-related quality of life, with a median MG-ADL score of 6 and a median MG-QOL15 score of 21. Seventy-one percent of the patients had received pyridostigmine. Corticosteroids, mycophenolate mofetil, and azathioprine were the most common immunomodulators/immunosuppressants, with 85% of participants having ever using one of these agents. Forty-seven registrants reported receiving intravenous immunoglobulin, and 30% received plasma exchange. Twelve percent reported other treatments, and 40% were unsure whether they received less common therapies. Forty percent had undergone thymectomy.
Discussion: The MGR data correlate well with other MG cohorts. Many MG patients remain negatively impacted despite treatment.
Keywords: ADL; MG; MGFA; QOL; myasthenia.
© 2019 The Authors. Muscle & Nerve published by Wiley Periodicals, Inc.
Conflict of interest statement
R.F.F. is an employee of Ra Pharmaceuticals, Inc, which funded the study. P.W.D. is an employee of Ra Pharmaceuticals. G.C. is employed by the University of Alabama at Birmingham and president of Pythagoras, Inc, a private consulting company in Birmingham, Alabama. G.C. is also a member of the data safety and monitoring boards for AMO Pharmaceuticals, Biolinerx, Horizon Pharmaceuticals, Hisun Pharmaceuticals, Merck, Merck/Pfizer, Opko Biologics, Neurim, Novartis, Ophazyme, Sanofi‐Aventis, Reata Pharmaceuticals, Receptos/Celgene, Teva Pharmaceuticals, the NHLBI (protocol review committee), and the NICHD (OPRU oversight committee), and is on the consulting or advisory boards of Atara Biotherapeutics, Axon, Biogen, Argenix, Brainstorm Cell Therapeutics, Charleston Labs, Click Therapeutics, Genzyme, Genentech, GW Pharma, Klein‐Buendel, Medimmune, Medday, Novartis, Roche, Scifluor, Somahlution, Teva Pharmaceuticals, TG Therapeutics, and University of Texas at Houston. H.J.K. is a member of the data safety and monitoring boards for Novartis and the National Institutes of Health; receives consulting fees from Alnylam Pharmaceuticals, UCB, Biocatalyst, RA Pharmaceuticals, and Momenta Pharmaceuticals; receives grant support from Akari Pharmaceuticals and the Muscular Dystrophy Association; and holds a patent related to technology to focus a complement inhibitor on the neuromuscular junction for the treatment of myasthenia gravis (US Patent No. 8,961,981). The remaining authors have no potential conflicts of interest to disclose related to this report.
Figures


References
-
- Berrih‐Aknin S, Le Panse R. Myasthenia gravis and autoantibodies: pathophysiology of the different subtypes. Rev Med Interne. 2014;35:413‐420. - PubMed
-
- Gilhus NE. Myasthenia gravis. N Engl J Med. 2017;376:e25. - PubMed
-
- Sussman J, Farrugia ME, Maddison P, Hill M, Leite MI, Hilton‐Jones D. Myasthenia gravis: Association of British Neurologists' management guidelines. Pract Neurol. 2015;15:199‐206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

