Treatment of Wilms Tumor in Sub-Saharan Africa: Results of the Second French African Pediatric Oncology Group Study
- PMID: 31487216
- PMCID: PMC6872179
- DOI: 10.1200/JGO.18.00204
Treatment of Wilms Tumor in Sub-Saharan Africa: Results of the Second French African Pediatric Oncology Group Study
Abstract
Purpose: Multidisciplinary management of Wilms tumor has been defined through multicenter prospective studies and an average expected patient cure rate of 90%. In sub-Saharan Africa, such studies are uncommon. After the encouraging results of the first Groupe Franco-Africain d'Oncologie Pédiatrique (GFAOP) study, we report the results of the GFAOP-NEPHRO-02 study using an adaptation of the International Society of Paediatric Oncology 2001 protocol.
Patients and methods: From April 1, 2005, to March 31, 2011, seven African units participated in a nonrandomized prospective study. All patients who were referred with a clinical and radiologic diagnosis of renal tumor were screened. Those older than age 6 months and younger than 18 years with a unilateral tumor previously untreated were pre-included and received preoperative chemotherapy. Patients with unfavorable histology or with a tumor other than Wilms, or with a nonresponding stage IV tumor were excluded secondarily.
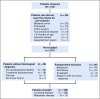
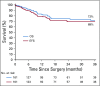
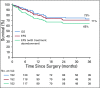
Results: Three hundred thirteen patients were initially screened. Two hundred fifty-seven patients were pre-included and 169 with histologic confirmation of intermediate-risk nephroblastoma were registered in the study and administered postoperative treatment. Thirty-one percent of patients were classified as stage I, 38% stage II, 24% stage III, and 7% stage IV. Radiotherapy was not available for any stage III patients. Three-year overall survival rate was 72% for all study patients and 73% for those with localized disease.
Conclusion: It was possible to conduct sub-Saharan African multicenter therapeutic studies within the framework of GFAOP. Survival results were satisfactory. Improvements in procedure, data collection, and outcome are expected in a new study. Radiotherapy is needed to reduce the relapse rate in patients with stage III disease.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
No potential conflicts of interest were reported.
Figures

References
-
- Breslow N, Olshan A, Beckwith JB, et al. Epidemiology of Wilms tumor. Med Pediatr Oncol. 1993;21:172–181. - PubMed
-
- Lemerle J, Voûte PA, Tournade MF, et al. Effectiveness of preoperative chemotherapy in Wilms’ tumor: Results of an International Society of Paediatric Oncology (SIOP) clinical trial. J Clin Oncol. 1983;1:604–609. - PubMed
-
- de Kraker J, Lemerle J, Voûte PA, et al. Wilm’s tumor with pulmonary metastases at diagnosis: The significance of primary chemotherapy. J Clin Oncol. 1990;8:1187–1190. - PubMed
-
- Tournade MF, Com-Nougué C, Voûte PA, et al. Results of the Sixth International Society of Pediatric Oncology Wilms’ Tumor Trial and Study: A risk-adapted therapeutic approach in Wilms’ tumor. J Clin Oncol. 1993;11:1014–1023. - PubMed
-
- Tournade MF, Com-Nougué C, de Kraker J, et al. Optimal duration of preoperative therapy in unilateral and nonmetastatic Wilms’ tumor in children older than 6 months: Results of the Ninth International Society of Pediatric Oncology Wilms’ Tumor Trial and Study. J Clin Oncol. 2001;19:488–500. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

