Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results From the Phase I/II CheckMate 358 Trial
- PMID: 31487218
- PMCID: PMC6823884
- DOI: 10.1200/JCO.19.00739
Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results From the Phase I/II CheckMate 358 Trial
Abstract
Purpose: Nivolumab was assessed in patients with virus-associated tumors in the phase I/II CheckMate 358 trial (ClinicalTrials.gov identifier: NCT02488759). We report on patients with recurrent/metastatic cervical, vaginal, or vulvar cancers.
Patients and methods: Patients received nivolumab 240 mg every 2 weeks. Although patients with unknown human papillomavirus status were enrolled, patients known to have human papillomavirus-negative tumors were ineligible. The primary end point was objective response rate. Duration of response (DOR), progression-free survival, and overall survival were secondary end points. Safety and patient-reported outcomes were exploratory end points.
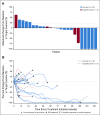
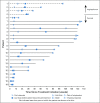
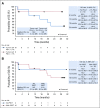
Results: Twenty-four patients (cervical, n = 19; vaginal/vulvar, n = 5) were enrolled. Most patients had received prior systemic therapy for metastatic disease (cervical, 78.9%; vaginal/vulvar, 80.0%). Objective response rates were 26.3% (95% CI, 9.1 to 51.2) for cervical cancer and 20.0% (95% CI, 0.5 to 71.6) for vaginal/vulvar cancers. At a median follow-up of 19.2 months, median DOR was not reached (range, 23.3 to 29.5+ months; + indicates a censored observation) in the five responding patients in the cervical cohort; the DOR was 5.0 months in the single responding patient in the vaginal/vulvar cohort. Median overall survival was 21.9 months (95% CI, 15.1 months to not reached) among patients with cervical cancer. Any-grade treatment-related adverse events were reported in 12 of 19 patients (63.2%) in the cervical cohort and all five patients in the vaginal/vulvar cohort; there were no treatment-related deaths. In the cervical cohort, nivolumab treatment generally resulted in stabilization of patient-reported outcomes associated with health status and health-related quality of life.
Conclusion: The efficacy of nivolumab in patients with recurrent/metastatic cervical and vaginal or vulvar cancers is promising and warrants additional investigation. No new safety signals were identified with nivolumab treatment in this population.
Figures


References
-
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. - PubMed
-
- Faber MT, Sand FL, Albieri V, et al. Prevalence and type distribution of human papillomavirus in squamous cell carcinoma and intraepithelial neoplasia of the vulva. Int J Cancer. 2017;141:1161–1169. - PubMed
-
- National Cancer Institute: Table 5.1. Cancer of the cervix uteri (invasive). https://seer.cancer.gov/csr/1975_2015/results_merged/sect_05_cervix_uter....
-
- Marth C, Landoni F, Mahner S, et al: Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv72-iv83, 2017 (suppl 4) - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

