Multi-component meningococcal serogroup B (MenB)-4C vaccine induces effective opsonophagocytic killing in children with a complement deficiency
- PMID: 31487400
- PMCID: PMC6857189
- DOI: 10.1111/cei.13368
Multi-component meningococcal serogroup B (MenB)-4C vaccine induces effective opsonophagocytic killing in children with a complement deficiency
Abstract
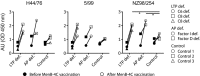
Vaccination against meningococcal serogroup B is recommended for patients with a complement deficiency; however, although immunogenicity in this patient group has been shown, efficacy has not yet been established. In this study, we collected serum from children with a complement deficiency in the alternative pathway or in late terminal pathway before and after vaccination with multi-component meningococcal serogroup B (MenB)-4C. MenB-4C is a multi-component, protein-based vaccine against MenB consisting of factor H-binding protein, Neisserial heparin-binding protein, Neisserial adhesion A and outer membrane vesicles containing Porin A. We assessed the vaccine immunogenicity and vaccine-mediated protection by a whole cell enzyme-linked immunosorbent assay with Neisseria meningitidis serogroup B strains H44/76, 5/99 and NZ98/254, which shows that vaccination induced antibody titers against meningococcus. We show that the classical serum bactericidal activity assay with exogenous serum indicates the presence of vaccine-induced antibodies and capacity to activate complement-mediated pathogen lysis. However, in children with a late terminal pathway deficiency, no complement-mediated pathogen lysis was observed when autologous serum was applied in the serum bactericidal activity assay, demonstrating a lack of serum bactericidal activity in children with complement deficiencies. However, MenB-4C vaccination still induced effective complement-dependent opsonophagocytic killing against N. meningitidis serogroup B in reconstituted whole blood with autologous serum from children with an alternative pathway or late terminal pathway deficiency. These findings support the recommendation to vaccinate all complement-deficient children against MenB.
Keywords: Neisseria meningitidis; MenB-4C; children; complement-deficient; vaccine.
© 2019 The Authors. Clinical & Experimental Immunology published by John Wiley & Sons Ltd on behalf of British Society for Immunology.
Conflict of interest statement
The authors declare that they have no conflicts of interest
Figures


References
-
- Skattum L, van Deuren M, van der Poll T, Truedsson L. Complement deficiency states and associated infections. Mol Immunol 2011; 48:1643–55. - PubMed
-
- Rosain J, Hong E, Fieschi C, Martins PV, El Sissy C, Deghmane AE. Strains responsible for invasive meningococcal disease in patients with terminal complement pathway deficiencies. J Infect Dis 2017; 215:1331–8. - PubMed
-
- Parikh SR, Andrews NJ, Beebeejaun K et al Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet 2016; 388:2775–82. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

