Modular Approach to Degradable Acetal Polymers Using Cascade Enyne Metathesis Polymerization
- PMID: 31487416
- PMCID: PMC7265103
- DOI: 10.1002/anie.201909172
Modular Approach to Degradable Acetal Polymers Using Cascade Enyne Metathesis Polymerization
Abstract
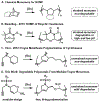
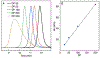
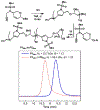
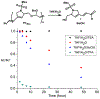
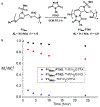
A modular synthetic approach to degradable metathesis polymers is presented using acetal-containing enyne monomers. The monomers are prepared in a short and divergent synthetic sequence that features two points of modification to tune polymerization behavior and introduce molecular cargo. Steric and stereochemical elements are critical in the monomer design in order to provide rapid and living polymerizations capable of generating block polymers. The developed polyacetal materials readily undergo pH-dependent degradation in aqueous mixtures, and the rate of hydrolysis can be tuned through post-polymerization modification with triazolinedione click chemistry. This presents a new scaffold for responsive metathesis polymers that may find use in applications that requires controllable breakdown and release of small molecules.
Keywords: acetal; degradable polymer; enyne monomers; living polymerization; metathesis polymerization.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
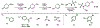
References
-
- Leitgeb A, Wappel J, Slugovc C, Polymer 2010, 51, 2927–2946.
-
- Smith D, Pentzer EB, Nguyen ST, Polym. Rev 2007, 47, 419–459
- Kanai M, Mortell KH, Kiessling LL, J. Am. Chem. Soc 1997, 119, 9931–9932
- Pohl NL, Kiessling LL, Synthesis 1999, 1999, 1515–1519
- Kammeyer JK, Blum AP, Adamiak L, Hahn ME, Gianneschi NC, Polym. Chem 2013, 4, 3929–3933 - PMC - PubMed
- James CR, Rush AM, Insley T, Vuković L, Adamiak L, Král P, Gianneschi NC, J. Am. Chem. Soc 2014, 136, 11216–11219 - PMC - PubMed
- Adamiak L, Touve MA, LeGuyader CLM, Gianneschi NC, ACS Nano 2017, 11, 9877–9888 - PubMed
- Lienkamp K, Madkour AE, Musante A, Nelson CF, Nüsslein K, Tew GN, J. Am. Chem. Soc 2008, 130, 9836–9843 - PMC - PubMed
- Isarov SA, Pokorski JK, ACS Macro Lett. 2015, 4, 969–973. - PubMed
-
- Atallah P, Wagener KB, Schulz MD, Macromolecules 2013, 46, 4735–4741
- Fokou PA, Meier MAR, J. Am. Chem. Soc 2009, 131, 1664–1665 - PubMed
- Lv A, Cui Y, Du F-S, Li Z-C, Macromolecules 2016, 49, 8449–8458
- Mutlu H, Barner-Kowollik C, Polym. Chem 2016, 7, 2272–2279
- Parkhurst RR, Balog S, Weder C, Simon YC, RSC Adv. 2014, 4, 53967–53974
- Khaja SD, Lee S, Murthy N, Biomacromolecules 2007, 8, 1391–1395. - PubMed
-
- Hodge P, Chem. Rev 2014, 114, 2278–2312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

