DREB/CBF expression in wheat and barley using the stress-inducible promoters of HD-Zip I genes: impact on plant development, stress tolerance and yield
- PMID: 31487424
- PMCID: PMC7004899
- DOI: 10.1111/pbi.13252
DREB/CBF expression in wheat and barley using the stress-inducible promoters of HD-Zip I genes: impact on plant development, stress tolerance and yield
Abstract
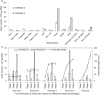
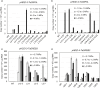
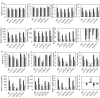
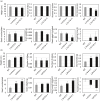
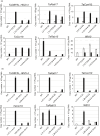
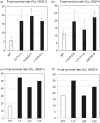
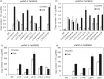
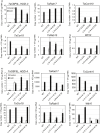
Networks of transcription factors regulate diverse physiological processes in plants to ensure that plants respond to abiotic stresses rapidly and efficiently. In this study, expression of two DREB/CBF genes, TaDREB3 and TaCBF5L, was modulated in transgenic wheat and barley, by using stress-responsive promoters HDZI-3 and HDZI-4. The promoters were derived from the durum wheat genes encoding the γ-clade TFs of the HD-Zip class I subfamily. The activities of tested promoters were induced by drought and cold in leaves of both transgenic species. Differences in sensitivity of promoters to drought strength were dependent on drought tolerance levels of cultivars used for generation of transgenic lines. Expression of the DREB/CBF genes under both promoters improved drought and frost tolerance of transgenic barley, and frost tolerance of transgenic wheat seedlings. Expression levels of the putative TaCBF5L downstream genes in leaves of transgenic wheat seedlings were up-regulated under severe drought, and up- or down-regulated under frost, compared to those of control seedlings. The application of TaCBF5L driven by the HDZI-4 promoter led to the significant increase of the grain yield of transgenic wheat, compared to that of the control wild-type plants, when severe drought was applied during flowering; although no yield improvements were observed when plants grew under well-watered conditions or moderate drought. Our findings suggest that the studied HDZI promoters combined with the DREB/CBF factors could be used in transgenic cereal plants for improvement of abiotic stress tolerance, and the reduction of negative influence of transgenes on plant development and grain yields.
Keywords: C-repeat binding factor 5-like protein; HDZI-4 and HDZI-3 promoters; Phenotype; transcription factors; transgenic barley and wheat.
© 2019 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
Authors declare no conflict of interest.
Figures

References
-
- Agarwal, P.K. , Gupta, K. , Lopato, S. and Agarwal, P. (2017) Dehydration responsive element binding transcription factors and their applications for the engineering of stress tolerance. J. Exp. Bot. 68, 2135–2148. - PubMed
-
- Altschul, S.F. , Gish, W. , Miller, W. , Myers, E.W. and Lipman, D.J. (1990) Basic local alignment search tool. J. Mol. Biol. 215, 403–410. - PubMed
-
- Ban, Q. , Liu, G. and Wang, Y. (2011) A DREB gene from Limonium bicolor mediates molecular and physiological responses to copper stress in transgenic tobacco. J. Plant Physiol. 168, 449–458. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

